沸石中 Ru 和 Mn 之间的亚纳米电子相互作用工程可促进二氯甲烷的催化氧化
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
设计具有活性和稳定性的催化剂仍然是催化氧化去除氯化挥发性有机化合物(CVOCs)的一个巨大挑战。本文合成了包封在ZSM-5沸石(RuMn@Z)中的Ru-Mn亚纳米物质。结果表明,二氯甲烷90%的转化率低至320℃,明显低于Ru@Z(350℃)和浸渍催化剂(RuMn/Z, 355℃)。重要的是,RuMn@Z催化剂具有优异的耐高温性能(800°C 10 h),耐水性,长期稳定性和循环稳定性。与RuMn/Z具有纳米尺度的金属相互作用不同,RuMn@Z中Ru和Mn由于沸石的约束作用在亚纳米尺度上具有较强的电子相互作用。Mn作为电子结构调节剂,通过Ru - o - Mn键使Ru保持缺电子状态,有效激活晶格氧和分子氧参与反应。此外,约束效应还使酸与氧化还原位点紧密偶联,促进脱氯产物、甲酸酯等中间产物的快速转化。因此,该研究为CVOCs去除和其他环境领域的高性能催化剂的设计提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
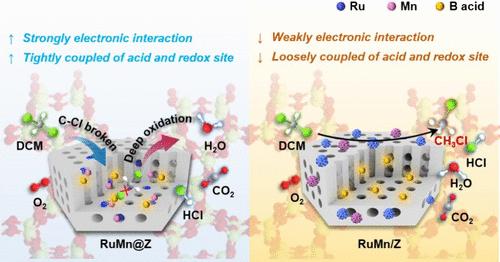
Engineering Subnanometric Electronic Interaction between Ru and Mn in Zeolite Boosts Catalytic Oxidation of Dichloromethane
Designing catalysts with both activity and stability remains a grand challenge for the removal of chlorinated volatile organic compounds (CVOCs) by catalytic oxidation. Herein, the Ru–Mn subnanometric species encapsulated in ZSM-5 zeolite (RuMn@Z) was synthesized. It shows that the 90% conversion of dichloromethane is as low as 320 °C, which is significantly lower than that of Ru@Z (350 °C) and the impregnation catalyst (RuMn/Z, 355 °C). Importantly, the RuMn@Z catalyst exhibits excellent high-temperature resistance (800 °C for 10 h), water resistance, long-term stability, and cyclic stability. Different from the RuMn/Z with nanoscale metal interaction, Ru and Mn species in RuMn@Z have strong electronic interaction on the subnanometric scale due to the confinement effect of zeolite. As the electronic structure regulator, the Mn species keeps the Ru species in an electron-deficient state through the Ru–O–Mn bonds, which effectively activates lattice oxygen species and molecular oxygen to participate in the reaction. Moreover, the confinement effect also makes the acid tightly coupled with the redox site, which promotes the rapid conversion of dechlorination products, formates, and other intermediate products. Therefore, this study provides valuable insights into the design of high-performance catalysts for CVOCs removal and other environmental fields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


