不同离子溶液中悬浮/负载石墨烯的石墨烯-离子界面的纳米级超级电容研究
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
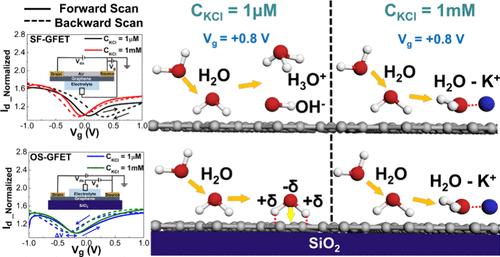
基于石墨烯的超级电容器由于其卓越的能量存储能力而获得了极大的关注。尽管有大量的研究努力试图提高性能,但实验阐明纳米级界面分子特性的挑战仍然需要解决,以便在商业应用中进行设备优化。为了解决这一问题,我们利用无衬底石墨烯场效应晶体管(sf - gfet)和氧化负载石墨烯场效应晶体管(os - gfet)进行了一系列实验,以阐明石墨烯-电解质界面排列和不同表面电位状态和离子浓度环境下的相应电容。对于SF-GFET,我们观察到随着离子浓度的增加,Dirac点的迟滞从0.32 V变化到- 0.06 V。界面电容由4 ~ 2 F/g变化。对于OS-GFET, Dirac点迟滞保持为负(- 0.15 ~ - 0.07 V),并且随着离子浓度的增加,OS-GFET的相应电容减小(53 ~ 16 F/g)。这表明石墨烯-水界面上有序取向的水结构逐渐被离子水化团簇所取代,从而导致了电容的差异。dirac点迟滞值与离子浓度之间的关系可以用一阶Hill方程进行建模,得到半占用值(KCl溶液K = 1.0131 × 10-4, MgCl2溶液K = 6.6237 × 10-5)。这也与两种矿物在界面内水层中离子水化的差异一致。这项工作说明了界面纳米级排列对界面电容形成的影响以及对超级电容器发展的布局意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nanoscopic Supercapacitance Elucidations of the Graphene-Ionic Interface with Suspended/Supported Graphene in Different Ionic Solutions
Graphene-based supercapacitors have gained significant attention due to their exceptional energy storage capabilities. Despite numerous research efforts trying to improve the performance, the challenge of experimentally elucidating the nanoscale-interface molecular characteristics still needs to be tackled for device optimizations in commercial applications. To address this, we have conducted a series of experiments using substrate-free graphene field-effect transistors (SF-GFETs) and oxide-supported graphene field-effect transistors (OS-GFETs) to elucidate the graphene-electrolyte interfacial arrangement and corresponding capacitance under different surface potential states and ionic concentration environments. For SF-GFET, we observed that the hysteresis of the Dirac point changes from 0.32 to −0.06 V as the ionic concentration increases. Moreover, it results in the interfacial capacitance changing from 4 to 2 F/g. For OS-GFET, the hysteresis of the Dirac point remains negative (−0.15 to −0.07 V). Furthermore, the corresponding capacitance of OS-GFET decreases (53–16 F/g) as the ionic concentration increases. These suggest that the orderly oriented water structure at the graphene–water interface is gradually replaced by ionic hydration clusters and results in the difference of capacitance. The relationship between Dirac-point hysteresis value and ionic concentration can be modeled by using the first-order Hill equation to obtain the half occupation value (K = 1.0131 × 10–4 for KCl solution and K = 6.6237 × 10–5 for MgCl2 solution). This also agrees with the variances of two minerals in ion hydration within the inner water layer at the interface. This work illustrates the influence of interfacial nanoscale arrangement on interface capacitance formation and layout implications for the development of supercapacitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


