同源重组促进DNA损伤后非免疫原性有丝分裂细胞死亡
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
双链断裂(DSB)可引发有丝分裂灾难,这是一种复杂的抑制现象,其特点是细胞在分裂过程中或分裂后死亡。在这里,我们通过单细胞延伸活体成像分析揭示了细胞周期调控的DSB修复如何引导不同的细胞死亡结果。在S期或G2期诱导DSB后,未解决的同源重组中间体进入有丝分裂期,在细胞分裂的直接尝试中促进非免疫原性的内在凋亡。相反,非同源末端连接、微同源介导的末端连接和单链退火相互配合,使受损的 G1 细胞以延迟的外源性致死和干扰素产生为代价,完成第一个细胞周期的异常细胞分裂。针对非同源末端连接、微同源介导的末端连接或单链退火会促进有丝分裂死亡,而抑制有丝分裂死亡则会增强干扰素的产生。这些数据共同表明,时间修复层次结构与累积的DSB负荷相结合,可以可靠地预测基因组损伤后有丝分裂的灾难性结果。在这一途径中,同源重组通过促进有丝分裂致死来抑制干扰素的产生。本文章由计算机程序翻译,如有差异,请以英文原文为准。
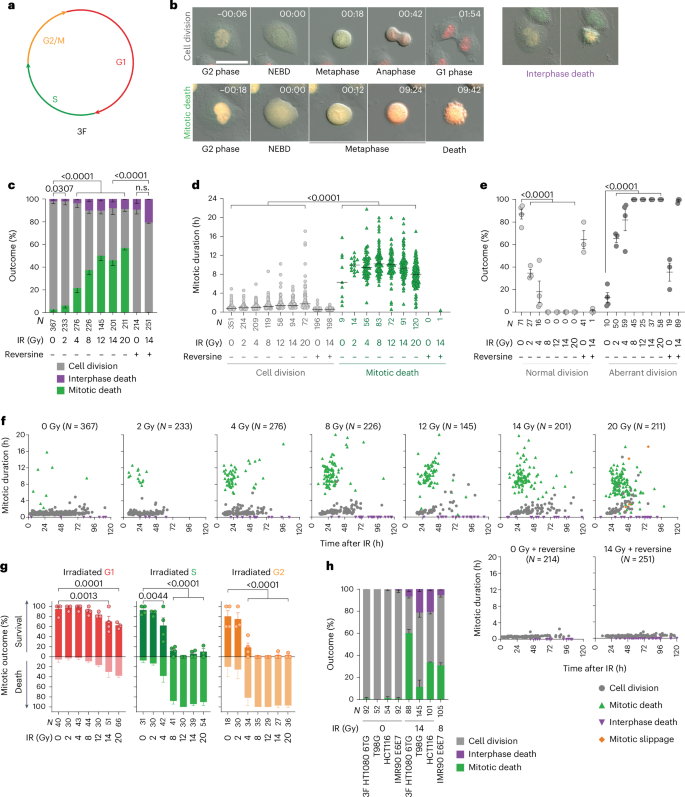

Homologous recombination promotes non-immunogenic mitotic cell death upon DNA damage
Double-strand breaks (DSBs) can initiate mitotic catastrophe, a complex oncosuppressive phenomenon characterized by cell death during or after cell division. Here we unveil how cell cycle-regulated DSB repair guides disparate cell death outcomes through single-cell analysis of extended live imaging. Following DSB induction in S or G2, passage of unresolved homologous recombination intermediates into mitosis promotes non-immunogenic intrinsic apoptosis in the immediate attempt at cell division. Conversely, non-homologous end joining, microhomology-mediated end joining and single-strand annealing cooperate to enable damaged G1 cells to complete the first cell cycle with an aberrant cell division at the cost of delayed extrinsic lethality and interferon production. Targeting non-homologous end joining, microhomology-mediated end joining or single-strand annealing promotes mitotic death, while suppressing mitotic death enhances interferon production. Together the data indicate that a temporal repair hierarchy, coupled with cumulative DSB load, serves as a reliable predictor of mitotic catastrophe outcomes following genome damage. In this pathway, homologous recombination suppresses interferon production by promoting mitotic lethality. Szmyd et al. show that DNA repair pathways impact whether cells with DNA lesions arrest in mitosis. The formation of homologous recombination-driven double Holliday junctions elicits mitotic cell death and suppresses inflammatory signalling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


