Pt(111)/全氟磺酸离聚体界面阳离子-电子耦合转移及其对氧还原反应动力学的影响
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
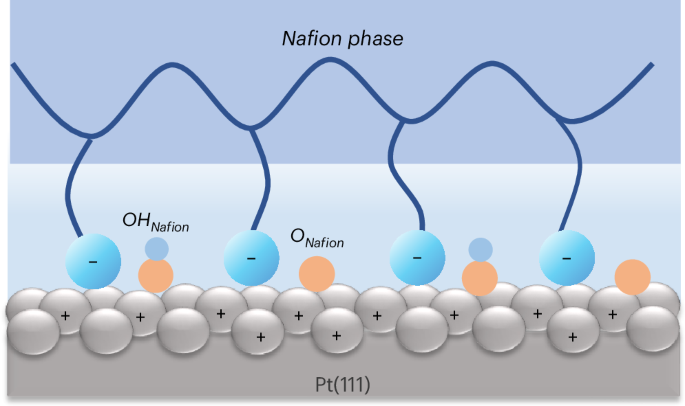
聚合物电解质和电极之间的电化学界面是全球向可再生能源过渡的电化学装置的核心。在这里,我们证明了磺酸盐在Pt(111)上的吸附和解吸涉及不同的基本步骤,后者通过一个耦合的阳离子-电子转移进行。吸附的磺酸盐不仅阻断了部分表面Pt位点,更重要的是,还生成了两种额外的表面吸附物,OHNafion和ONafion,它们分别与裸Pt(111)上吸附的OH和O表现出不同的动力学性质。现有的热力学描述符无法解释Nafion中磺酸基对Pt上氧还原反应(ORR)活性的影响。Pt(111)表面的ORR活性降低是由于吸附的磺酸盐附近的*O→*OH转化和*OH还原受到动力学阻碍所致。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Coupled cation–electron transfer at the Pt(111)/perfluoro-sulfonic acid ionomer interface and its impact on the oxygen reduction reaction kinetics
Electrochemical interfaces between polymer electrolytes and electrodes are central to electrochemical devices in the global transition towards renewable energy. Here we show that the adsorption and desorption of sulfonates in Nafion on Pt(111) involve distinct elementary steps, with the latter proceeding through a coupled cation–electron transfer. Adsorbed sulfonates not only block a fraction of surface Pt sites but, more importantly, generate two additional types of surface adsorbate, OHNafion and ONafion, which exhibit distinct kinetic properties from adsorbed OH and O on bare Pt(111), respectively. The impact of the adsorption of sulfonate groups in Nafion on the activity of the oxygen reduction reaction (ORR) on Pt cannot be rationalized by existing thermodynamic descriptors. The reduced ORR activity on the Nafion-covered Pt(111) is caused by the kinetically hindered *O→*OH conversion and *OH reduction on sites close to adsorbed sulfonates. The understanding of electrochemical interfaces between polymer electrolytes and metal electrodes, which is critical to many practical devices, remains limited. Now, the interaction between Nafion’s sulfonate groups and platinum and its impact on the oxygen reduction reaction is studied in detail, and a distinct coupled cation–electron transfer mechanism is identified.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


