通过液-液相分离组装的凝聚囊泡改善了生物药物的输送
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
囊泡在细胞物质储存和信号运输中起着至关重要的作用,甚至在细胞器和细胞的形成中也起着重要作用。天然囊泡由脂质层组成,脂质层形成一层膜,将内部物质包裹起来。在这里,我们报告了一个凝聚囊泡形成的液-液相分离胆固醇修饰的DNA和组蛋白。与基于磷脂的膜结合囊泡不同,凝聚囊泡表面缺乏膜结构,由高密度的液体层和充满水的空腔组成。通过一个简单的凝聚过程,我们证明了各种生物制剂,包括病毒颗粒、mRNA、细胞因子和肽,可以在液相中无害地直接富集。与易于聚集挑战的液滴状凝聚体相比,凝聚体囊泡表现出优越的动力学稳定性,使其成为生物制药的多功能运载工具。我们在小鼠模型中证实,将溶瘤病毒整合到这些凝聚囊泡中,使它们具有强大的溶瘤功效,并引发强大的抗肿瘤免疫反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。

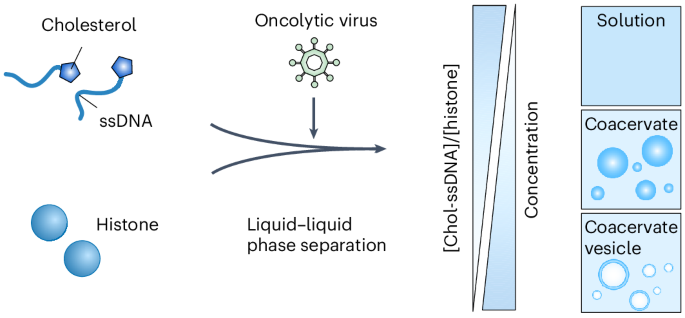
Coacervate vesicles assembled by liquid–liquid phase separation improve delivery of biopharmaceuticals
Vesicles play critical roles in cellular materials storage and signal transportation, even in the formation of organelles and cells. Natural vesicles are composed of a lipid layer that forms a membrane for the enclosure of substances inside. Here we report a coacervate vesicle formed by the liquid–liquid phase separation of cholesterol-modified DNA and histones. Unlike a phospholipid-based membrane-bounded vesicle, a coacervate vesicle lacks a membrane structure on the surface and is organized with a high-density liquid layer and a water-filled cavity. Through a straightforward coacervation process, we demonstrate that various biological agents, including virus particles, mRNA, cytokines and peptides, can be innocuously and directly enriched in the liquid phase. In contrast to the droplet-like coacervates that are prone to aggregation challenges, coacervate vesicles display superior kinetic stability, positioning them as a versatile delivery vehicle for biopharmaceuticals. We validate that incorporating oncolytic viruses into these coacervate vesicles endows them with potent oncolytic efficacy and elicits robust anti-tumour immune responses in mouse models. Natural vesicles typically consist of a lipid membrane enclosing substances. Now a coacervate vesicle formed by liquid–liquid phase separation of cholesterol-modified DNA and histones has been developed. Unlike traditional vesicles, these lack a membrane and feature a high-density liquid layer around a water-filled cavity, offering enhanced kinetic stability and potential as a biopharmaceutical delivery system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


