通过可重构DNA纳米工艺合成细胞的形态重塑和膜通道形成
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
生物物质的形状对不同长度尺度的细胞功能至关重要,并决定了细胞成分如何识别、相互作用和相互反应。然而,它们的形状往往是短暂的,很难重新编程。在此,我们构建了一个由信号响应DNA纳米细胞、生物孔和巨大单层囊泡(GUVs)组成的合成细胞模型。我们证明,DNA筏在纳米尺度上的重塑可以耦合到guv在微观尺度上的重塑。这些纳米粒子在脂质膜上共同经历了各向同性和短程局部秩序之间的可逆转变,可编程地重塑了GUV的形状。在生物孔隙的帮助下,在GUV形状恢复过程中,局部有序的DNA筏穿过膜,形成可密封的合成通道,用于大型货物运输。我们的工作概述了一个多功能平台,用于将可重构DNA纳米结构与合成细胞连接,扩大DNA纳米技术在合成生物学中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。

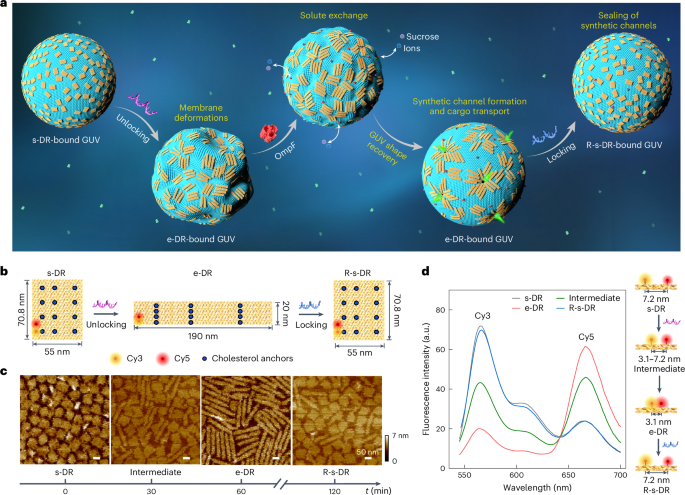
Morphology remodelling and membrane channel formation in synthetic cells via reconfigurable DNA nanorafts
The shape of biological matter is central to cell function at different length scales and determines how cellular components recognize, interact and respond to one another. However, their shapes are often transient and hard to reprogramme. Here we construct a synthetic cell model composed of signal-responsive DNA nanorafts, biogenic pores and giant unilamellar vesicles (GUVs). We demonstrate that reshaping of DNA rafts at the nanoscale can be coupled to reshaping of GUVs at the microscale. The nanorafts collectively undergo reversible transitions between isotropic and short-range local order on the lipid membrane, programmably remodelling the GUV shape. Assisted by the biogenic pores, during GUV shape recovery the locally ordered DNA rafts perforate the membrane, forming sealable synthetic channels for large cargo transport. Our work outlines a versatile platform for interfacing reconfigurable DNA nanostructures with synthetic cells, expanding the potential of DNA nanotechnology in synthetic biology. The shape of biological matter is central to their function and interaction with other cellular components. A combination of DNA origami nanorafts with biogenic pores reversibly controls the shape and permeability of lipid vesicles at the microscale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


