等位基因转录组学分析鉴定了prd样同源盒基因在人类胚胎裂解期阻滞中的作用
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
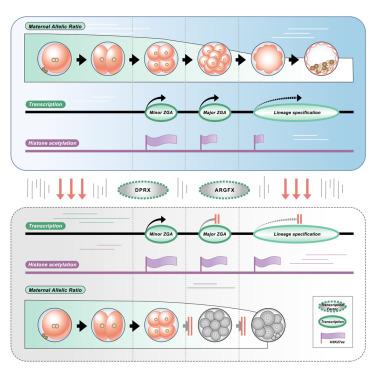
人类胚胎的卵裂期阻滞极大地限制了不孕症治疗的成功率,母体到受精卵过渡(MZT)异常是一个潜在的因素。然而,潜在的机制和监管机构仍不清楚。在这里,通过对人类着床前胚胎进行等位基因转录组分析,我们通过等位基因比例准确地量化了MZT的进展,并确定了一小部分8细胞胚胎,在适当的发育时间点,表现出正常的形态,处于转录阻滞状态。此外,我们确定了配对(PRD)样同源盒转录因子发散配对相关同源盒(DPRX)和精氨酸- 50同源盒(ARGFX)是参与MZT的因子,其缺乏严重损害MZT和谱系规范,并导致组蛋白乙酰化的异常保留。通过逆转由DPRX和ARGFX缺陷引起的乙酰化滞留,可以部分挽救胚胎骤停。我们的研究确定了与人类MZT有关的因素,并阐明了人类卵裂期阻滞的病因学。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Allelic transcriptomic profiling identifies the role of PRD-like homeobox genes in human embryonic-cleavage-stage arrest
Cleavage-stage arrest in human embryos substantially limits the success rate of infertility treatment, with maternal-to-zygotic transition (MZT) abnormalities being a potential contributor. However, the underlying mechanisms and regulators remain unclear. Here, by performing allelic transcriptome analysis on human preimplantation embryos, we accurately quantified MZT progression by allelic ratio and identified a fraction of 8-cell embryos, at the appropriate developmental time point and exhibiting normal morphology, were in transcriptionally arrested status. Furthermore, we identified PAIRED (PRD)-like homeobox transcription factors divergent paired-related homeobox (DPRX) and arginine-fifty homeobox (ARGFX) as factors involved in MZT, whose deficiency severely impairs MZT and lineage specification and leads to aberrant retention of histone acetylation. By reversing the acetylation retention caused by DPRX and ARGFX defects, embryonic arrest can be partially rescued. Our study identifies factors involved in human MZT and elucidates the etiology underlying human cleavage-stage arrest.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


