快速CO2偶联到丙炔醇:通过连续流动解锁α-烷基烯环碳酸酯的生产
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
α-烷基环碳酸盐(α - cc)作为有机化学和聚合物化学的基础材料,越来越受到人们的关注。迄今为止,它们的合成通过CO2偶联到丙炔醇已被限制在间歇过程,广泛的努力致力于改善催化系统。在此,我们利用一种精制的、均相的银-碳-有机碱催化体系,优化了间歇条件,首次在几分钟内实现叔丙醇的完全转化,而不是几小时。在此基础上,我们引入了一种连续流方法来生成α - cc库,实现了所报道的最高时空产量,在不到20分钟的时间内实现了定量转换,每天的产量高达111克。这种方法将二氧化碳的使用量减少到1或2个等价物,改善了参数控制,并有望促进可扩展性。此外,“即插即用”实验室规模的连续流程模块可以无缝集成后续αCC转化,而无需中间纯化,αCC氨解成恶唑酮的转化率为91%。此外,在聚合物基质上支持银-碳催化剂消除了银污染,甚至抑制了对碱助催化剂的需求。这项工作推进了α - ccs的可扩展合成,标志着向更绿色、基于二氧化碳的环状碳酸盐及其衍生物迈出了重要的一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
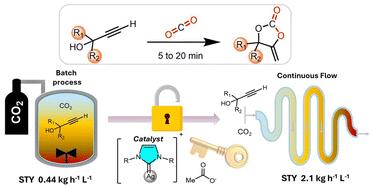
Rapid CO2 coupling to propargylic alcohols: unlocking the production of α-alkylidene cyclic carbonates via continuous flow†
α-Alkylidene cyclic carbonates (αCCs) are gaining interest as building blocks in organic and polymer chemistry. To date, their synthesis via the coupling of CO2 to propargylic alcohols has been restricted to batch processes, with extensive efforts devoted to improving catalytic systems. Herein, utilizing a refined, homogeneous silver–carbene–organobase catalytic system, we optimized batch conditions to achieve, for the first time, complete conversion of tertiary propargylic alcohols within minutes instead of hours. Building on this, we introduce a continuous flow methodology to produce a library of αCCs, achieving the highest space–time yields reported, with quantitative conversions in less than 20 minutes and outputs up to 111 grams per day. This approach reduces CO2 usage to 1 or 2 equivalents, improves parameter control, and is expected to facilitate scalability. In addition, “plug-and-play” lab-scale continuous flow modules enable seamless integration of subsequent αCC transformations without intermediate purification, as illustrated by the aminolysis of αCCs into oxazolidones with good conversion (91%). Furthermore, supporting the silver–carbene catalyst on a polymer matrix eliminates silver contamination and even suppresses the need for a base co-catalyst. This work advances the scalable synthesis of αCCs via continuous flow, marking a significant step toward greener, CO2-based cyclic carbonates and derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


