缺氧诱导的FTO降解通过揭开rack1介导的MTK1-JNK1/2通路的激活来促进细胞凋亡
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
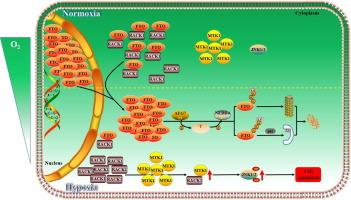
缺氧是一种以组织供氧不足为特征的状态,可引发各种细胞反应,包括细胞凋亡。RNA去甲基化酶FTO已被证明发挥抗凋亡作用,但其功能独立于RNA去甲基化酶-特别是那些涉及蛋白质-蛋白质相互作用-在缺氧期间仍不清楚。目的探讨FTO对缺氧应激下细胞凋亡的保护机制。方法在缺氧(1 % O2)条件下培养snh /3T3细胞、MEF细胞和小鼠颗粒细胞,并用抑制剂(氯喹、MG132、环己亚胺)处理FTO降解途径。利用RNA干扰敲除at7、nedd4和fto。质谱法鉴定了FTO相关蛋白,并用免疫沉淀法分析了它们与FTO的相互作用。通过核、细胞质分离和荧光显微镜检测FTO定位。流式细胞术(annexin V/PI)检测细胞凋亡。通过使用FB23-2或缺乏m6A去甲基化酶活性的228a /D230A突变体抑制FTO功能来确定FTO独立于m6A去甲基化酶活性的作用。结果缺氧暴露后,FTO在NIH/3T3细胞、MEF细胞和小鼠颗粒细胞中通过e1样泛素活化酶ATG7和E3泛素连接酶NEDD4协同激活泛素-蛋白酶体系统(UPS)和自噬-溶酶体途径(ALP)的调控途径从细胞核转移到细胞质中降解。此外,敲除atg7导致FTO在细胞质中积累,FTO通过与RACK1结合发挥保护作用,破坏RACK1与MTK1之间的相互作用,从而阻断JNK1/2的激活,从而阻止缺氧细胞的凋亡。本研究揭示了细胞质FTO在缺氧时破坏rack1 - mtk1 - jnk1 /2凋亡级联中的新功能,将FTO的功能背景定位在蛋白-蛋白相互作用层,这将其机制作用扩展到RNA去甲基化之外。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hypoxia-induced degradation of FTO promotes apoptosis by unmasking RACK1-mediated activation of MTK1-JNK1/2 pathway
Introduction
Hypoxia, a condition characterized by inadequate oxygen supply to tissues, triggers various cellular responses, including apoptosis. The RNA demethylase FTO has been shown to exert anti-apoptotic effects, but its functions independent of RNA demethylase—particularly those involving protein–protein interactions—during hypoxia remain unclear.Objectives
This study aimed to elucidate the cytoprotective mechanism of FTO in preventing apoptosis under hypoxic stress.Methods
NIH/3T3 cells, MEF cells, and mouse granulosa cells were cultured under hypoxia (1 % O2) and treated with inhibitors (chloroquine, MG132, cycloheximide) to identify FTO degradation pathways. RNA interference was used to knock down atg7, nedd4, and fto. Mass spectrometry identified FTO-associated proteins, and their interactions with FTO were analyzed with immunoprecipitation assays. FTO localization was examined through nuclear and cytoplasmic fractionation and fluorescence microscopy. Apoptosis was evaluated by flow cytometry (annexin V/PI). The role of FTO independent of its m6A demethylase activity was determined by inhibiting FTO function using FB23-2 or an H228A/D230A mutant lacking m6A demethylase activity.Results
Upon hypoxia exposure, FTO relocated from the nucleus to the cytoplasm and underwent degradation through a regulatory pathway in which the E1-like ubiquitin-activating enzyme ATG7 and the E3 ubiquitin ligase NEDD4 cooperatively activated both the ubiquitin–proteasome system (UPS) and the autophagic-lysosomal pathway (ALP) in NIH/3T3 cells, MEF cells, and mouse granulosa cells. Furthermore, knocking down atg7 resulted in FTO accumulation in the cytoplasm, where FTO exerted its protective effect by binding with RACK1, which impairs the interaction between RACK1 and MTK1, thereby blocking activation of JNK1/2 and subsequently preventing apoptosis in hypoxic cells.Conclusion
This study reveals a novel function of cytoplasmic FTO in disrupting the RACK1-MTK1-JNK1/2-apoptosis cascade during hypoxia, positioning the functional context of FTO at the layer of protein–protein interactions, which extends its mechanistic role beyond RNA demethylation.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


