一步恒流电解从废锂离子电池中选择性分离锂
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
从废锂离子电池(LIBs)中分离锂是能源可持续发展和资源节约的关键挑战。在这项研究中,我们提出了一种新的一步恒流电解(CCE)工艺,用于从废三元正极材料中选择性分离和回收锂,特别是lini0.76 co0.22 al0.020 o2 (NCA)。在优化条件下(电流密度为20 a·m−2,电解液浓度为0.05 mol·L-1 Na2SO4,电化学分离时间为120 min),锂离子选择性分离率为99.4 %。同时,高电流效率(94.7 %)和低能耗(39.1 Wh·mol−1)突出了该工艺的经济和环境优势。这种方法也可以成功地扩展到其他正极材料,如LiNi0.5Co0.3Mn0.2O2 (NCM),具有锂离子(Li+)的高选择性分离率。CCE工艺的有效性归因于正极材料的有序开放的三元层状结构,在适当的电解条件下可以促进Li+的释放。至关重要的是,在电解过程中,过渡金属元素镍和钴合金的浸出程度最低。镍(Ni3+)转化为Ni(OH)2,钴(Co2+)氧化为Co3+,在Li+释放后保持电荷平衡。这种简化且可扩展的方法代表了分离和净化技术的重大进步,为从废锂中回收锂提供了可持续的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。

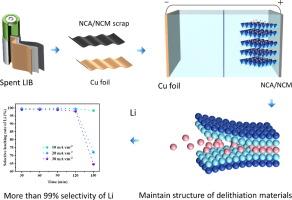
One-step constant current electrolysis for selective lithium separation from spent lithium-ion batteries
The separation of lithium from spent lithium-ion batteries (LIBs) is a critical challenge for sustainable energy development and resource conservation. In this study, we proposed a novel one-step constant current electrolysis (CCE) process for selective separation and recovery of lithium from spent ternary cathode materials, specifically LiNi0.76Co0.22Al0.02O2 (NCA). The developed process achieved a selective lithium separation of 99.4 % under the optimized conditions (with the current density of 20 A·m−2 and electrolyte concentration of 0.05 mol·L-1 Na2SO4 for an electrochemical separation duration of 120 min). Meanwhile, the high current efficiency (94.7 %) and low energy consumption (39.1 Wh·mol−1) highlight the economic and environmental advantages of the process. This approach can also be successfully extended to other cathode materials, such as LiNi0.5Co0.3Mn0.2O2 (NCM), with a high selective separation rate of lithium ions (Li+). The effectiveness of the CCE process is attributed to an orderly opened ternary layered structure of the cathode materials, which can facilitate the release of Li+ under appropriate electrolysis conditions. Crucially, the transition metal elements, nickel and cobyalt, were minimally leached during the electrolysis process. Nickel (Ni3+) was transformed into Ni(OH)2, and cobalt (Co2+) was oxidized to Co3+, maintaining the charge balance after the release of Li+. This streamlined and scalable approach represents a significant advancement in separation and purification technology, offering a sustainable solution for lithium recovery from spent LIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


