金属-有机接触中未占据分子轨道的受保护电子结构
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
分子电子性质在与金属原子成键后容易发生改变,这在很大程度上阻碍了分子电子器件的设计和工程。本文利用低温扫描隧道显微镜/光谱(STM/STS)研究了金属-有机接触中未占据分子轨道的受保护电子结构。在Au(11 11)上,双氰乙烯基六噻吩(DCV6T)分子自组装成各种纳米结构,包括Au原子配位链,其中轨道重新排列和重新分配是由Au配体杂化引起的。相反,当钴原子先于DCV6T沉积在Au(11 11)上时,形成共原子配位链。与钴原子的杂化导致了配体上的带隙态,这可能是由于钴三维态和占据的分子轨道的混合引起的。然而,STS测量表明,在与Co原子成键的DCV6T中,最低未占据分子轨道(LUMO)和LUMO + 1在轨道空间分布和能量排列方面表现出与非配位分子相同的特征。我们的研究表明,可以通过调整金属/配体组合来实现金属有机接触中所需轨道结构的保护。本文章由计算机程序翻译,如有差异,请以英文原文为准。
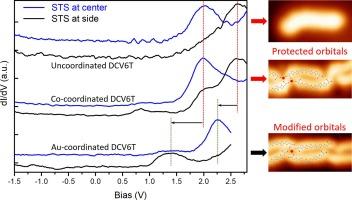
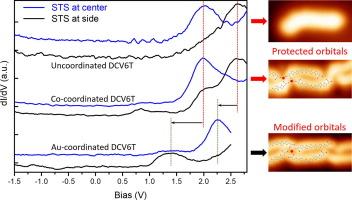
Protected electronic structures of unoccupied molecular orbitals in metal-organic contacts
Molecular electronic properties are prone to be modified upon bonding with metal atoms, which, to a large extent, hinders the design and engineering of molecular electronic devices. Here, we report on protected electronic structures of unoccupied molecular orbitals in metal–organic contacts, investigated by using low temperature scanning tunneling microscopy/spectroscopy (STM/STS). On Au(111), Dicyanovinyl-hexathiophene (DCV6T) molecules self-assemble into diverse nanostructures including Au-atom-coordinated chains, where orbital realignment and redistribution are induced by Au-ligand hybridization. When, instead, cobalt atoms are deposited on Au(111) previous to DCV6T deposition, Co-atom-coordinated chains are formed. The hybridization with Co atoms results in band gap states at the ligands, presumably caused by the mixing of the cobalt 3d states and the occupied molecular orbitals. Whereas, STS measurements resolve that the lowest unoccupied molecular orbital (LUMO) and LUMO + 1 show the same features in DCV6T bonding with Co atoms as those in the uncoordinated molecules, in terms of orbital spatial distribution and energy alignment. Our study demonstrates that the protection of desired orbital structures in metal–organic contacts could be achieved by tuning the metal/ligand combination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


