新型3-磺胺双尾吡咯-2- 1桥接分子作为有效的人类碳酸酐酶异构体抑制剂:设计、合成、分子模拟研究和MeWo、SK-BR-3和MG-63细胞系的抗癌活性
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
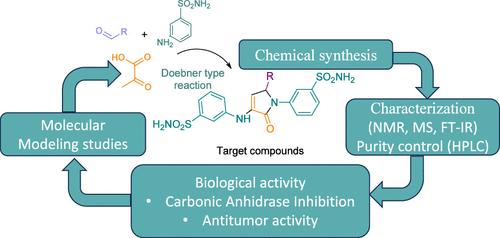
以三氟乙酸为催化剂,采用一锅三组分法合成了含有两个磺酰胺基团的新型3-磺酰胺吡咯-2- 1衍生物。利用光谱技术进行了结构确认。这些化合物对四种人类碳酸酐酶同工型(hCA I、hCA II、hCA IX和hCA XII)进行了测试,大多数衍生物对hCA II表现出显著的选择性,其中4h、4i、4n、4k和4j由于甲氧基和羟基模式而表现出增强的活性。化合物40对hcaii和hcaix具有较强的双选择性,而化合物41对hcaxii的抑制效果最好。此外,4e表现出对hCA XII抑制的偏好。对MeWo、SK-BR-3和MG-63癌细胞的生物学评价表明,化合物4l对MeWo细胞具有细胞毒性,但不显著影响正常成纤维细胞的活力。化合物4e、4l和40与阿霉素联用对MeWo癌细胞的活性有影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel 3-Sulfonamide Dual-Tail Pyrrol-2-one Bridged Molecules as Potent Human Carbonic Anhydrase Isoform Inhibitors: Design, Synthesis, Molecular Modeling Investigation, and Anticancer Activity in MeWo, SK-BR-3, and MG-63 Cell Lines
Novel 3-sulfonamide pyrrol-2-one derivatives containing two sulfonamide groups were synthesized via a one-pot, three-component method using trifluoroacetic acid as a catalyst. Structural confirmation was achieved using spectroscopic techniques. The compounds were tested against four selected human carbonic anhydrase isoforms (hCA I, hCA II, hCA IX, and hCA XII). Most derivatives showed significant selectivity for hCA II, with 4h, 4i, 4n, 4k, and 4j demonstrating enhanced activity due to methoxy and hydroxy group patterns. Compound 4o exhibited strong dual selectivity for hCA II and hCA IX, while 4l was the most effective inhibitor of hCA XII. Additionally, 4e showed a preference for hCA XII inhibition. Biological evaluation on MeWo, SK-BR-3, and MG-63 cancer cells showed that compound 4l was cytotoxic for MeWo cells without significantly affecting normal fibroblasts’ viability. Compounds 4e, 4l, and 4o were shown to affect tumor cell viability in combination with doxorubicin in additional testing on MeWo cancer cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


