氯消毒过程中多氟烷基物质转化途径的研究
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
最近关于饮用水中全氟化合物的法规强调,需要更深入地了解在氯消毒过程中由多氟烷基物质形成的全氟化合物。在本研究的化合物中,N-(3-(二甲基氨基丙烷-1-酰基)全氟-1-己胺磺酰胺(N- ap - fhxsa)在氯化过程中发生了快速转化。在一小时内,它可以定量生产各种多氟和全氟产品,包括全氟己酸(PFHxA)。研究了氯与N-AP-FHxSA及其季铵类似物的16种反应;其中7个被证实,其余的要么被否定,要么被认为无关紧要。季铵部分不能决定多氟烷基物质对氯的反应性。例如,虽然6:2氟端聚体磺胺甜菜碱能迅速转化为PFHxA,但其他含季铵盐的多氟烷基物质,如5:1:2和5:3氟端聚体甜菜碱,对氯化反应表现出明显的抗性。进一步的研究通过检查多氟烷基物质的最高占位分子轨道,确定了胺区附近亲电攻击的潜在位点。可视化技术分别帮助确定了亲核和亲电攻击的缺电子和富电子位点的潜在目标。将溶液pH值从6增加到10并没有减少所研究的多氟烷基物质的明显降解,这可能是由于与共轭酸相比,去质子化形式的反应性更强。最后,我们还研究了多氟烷基物质在pH为6至11时在无氯条件下的水解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
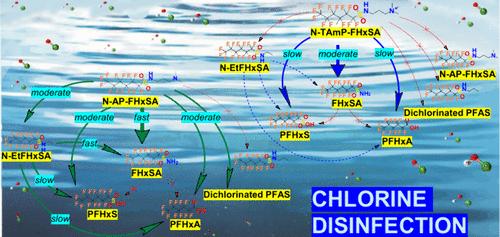
Investigation of Transformation Pathways of Polyfluoroalkyl Substances during Chlorine Disinfection
Recent regulations on perfluorinated compounds in drinking water underscore the need for a deeper understanding of the formation of perfluorinated compounds from polyfluoroalkyl substances during chlorine disinfection. Among the compounds investigated in this study, N-(3-(dimethylaminopropan-1-yl)perfluoro-1-hexanesulfonamide (N-AP-FHxSA) underwent rapid transformation during chlorination. Within an hour, it produced quantitative yields of various poly- and per-fluorinated products, including perfluorohexanoic acid (PFHxA). Sixteen reactions involving chlorine with N-AP-FHxSA and its quaternary ammonium analog were investigated; seven were confirmed, while the remainder were either disproved or found to be insignificant. The quaternary ammonium moiety did not determine a polyfluoroalkyl substance’s reactivity toward chlorine. For example, while 6:2 fluorotelomer sulfonamide betaine transformed rapidly to PFHxA, other quaternary-ammonium-containing polyfluoroalkyl substances, such as 5:1:2 and 5:3 fluorotelomer betaines, showed significant resistance to chlorination. Further investigation identified potential sites for electrophilic attacks near the amine region by examining the highest occupied molecular orbitals of the polyfluoroalkyl substances. Visualization techniques helped pinpoint electron-deficient and electron-rich sites as potential targets for nucleophilic and electrophilic attacks, respectively. Increasing the solution pH from 6 to 10 did not diminish the apparent degradation of the studied polyfluoroalkyl substances, likely due to the greater reactivity of the deprotonated forms compared to the conjugate acids. Finally, we also examined the hydrolysis of polyfluoroalkyl substances at pH 6 to 11 in the absence of chlorine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


