肝素与艰难梭菌毒素相互作用的特性及其作为抗cdi治疗药物的潜力。
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
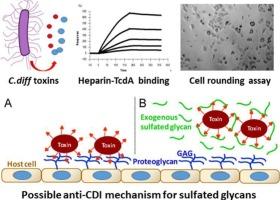
艰难梭菌(C. difficile)感染(CDI)是世界范围内发生的危及生命的卫生保健相关感染。艰难梭菌毒素(毒素A和毒素B)是主要的毒力因子,引起cdi相关腹泻和并发症。最近的研究表明,硫酸化糖胺聚糖(GAGs)参与介导这些毒素的细胞进入。虽然有报道称GAGs与毒素相互作用,但它们的结合动力学和促进毒素相互作用的聚糖的结构特征尚未得到深入研究。本研究利用表面等离子体共振(SPR)直接测量肝素与各种毒素相互作用的动力学。毒素A和毒素B与肝素具有高亲和力(KD分别为3.3 nM和13.5 nM)。SPR竞争试验表明,毒素A和毒素B都倾向于与较长的肝素链结合,肝素链上的所有硫酸化对于肝素-毒素相互作用至关重要。最后,体外实验表明,肝素和非抗凝肝素抑制毒素A引起的HeLa细胞的细胞圆缩。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Characterization of heparin interactions with Clostridioides difficile toxins and its potential as anti-CDI therapeutics
Clostridioides difficile (C. difficile) infection (CDI) is a life-threatening healthcare-associated infection occurring worldwide. C. difficile toxins (toxin A and toxin B) are the major virulence factors, causing CDI-related diarrhea and complications. Recent studies have shown that sulfated glycosaminoglcans (GAGs) are involved in mediating the cellular entry of these toxins. Although interactions between GAGs and toxins were reported, their binding kinetics and the structure features of glycans that facilitate toxin interaction have not been thoroughly studied. This research utilized surface plasmon resonance (SPR) to directly measure the kinetics of interactions between heparin and various toxins. Both toxin A and toxin B bind to heparin with high affinity (KD = 3.3 nM and 13.5 nM, respectively). SPR competition assay showed that both toxin A and B prefer binding to longer heparin chains and that all sulfation on the heparin chain is crucial for the heparin-toxin interaction. Finally, an in vitro assay showed that heparin and non-anticoagulant heparin inhibit the cell rounding caused by toxin A in HeLa cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


