用纳米铝佐剂拴系抗原mRNA人工标记肿瘤,招募并激活抗原特异性细胞毒性T细胞,以增强癌症免疫治疗。
IF 12.9
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
由于肿瘤表面抗原丢失和酸性肿瘤微环境(TME)失活导致免疫脱靶攻击,T细胞治疗实体瘤面临重大挑战。在此,我们开发了一种双功能免疫调节剂(MO@NAL),通过加载卵清蛋白(OVA;模型抗原mRNA (mOVA)在溶菌酶包被的层状双氢氧纳米铝佐剂(NA)上的表达。NA固有的碱性能有效中和TME内过量的酸,抑制调节性T细胞,为增强肿瘤细胞毒性T细胞浸润和活化创造有利的微环境。特别是,一旦被肿瘤细胞内化,MO@NAL通过携带的mOVA有效地用OVA标记肿瘤细胞表面,为募集和指导抗原特异性细胞毒性T细胞破坏肿瘤细胞提供靶标。在预先接种OVA疫苗的小鼠中,瘤内注射MO@NAL可迅速唤醒OVA特异性免疫记忆,快速有效地抑制早期和晚期结肠肿瘤和黑色素瘤的进展。在未预先接种疫苗的小鼠中,将MO@NAL与OVA治疗性疫苗或OVA特异性过继T细胞输注联合使用同样可以实现强大的实体肿瘤抑制。因此,这些发现强调了MO@NAL作为一种有效和安全的免疫调节剂的潜力,可以增强细胞毒性T细胞反应并及时干预实体瘤的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
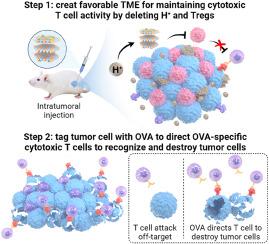
Artificially tagging tumors with nano-aluminum adjuvant-tethered antigen mRNA recruits and activates antigen-specific cytotoxic T cells for enhanced cancer immunotherapy
T cell therapy for solid tumors faces significant challenges due to the immune off-target attack caused by the loss of tumor surface antigens and inactivation in acidic tumor microenvironment (TME). Herein, we developed a bifunctional immunomodulator (MO@NAL) by loading ovalbumin (OVA; model antigen) mRNA (mOVA) onto lysozyme-coated layered double hydroxide nano-aluminum adjuvant (NA). The NA's inherent alkalinity effectively neutralizes the excess acid within the TME and suppresses regulatory T cells, creating a favorable microenvironment to enhance cytotoxic T cell infiltration and activation in tumors. Particularly, once internalization by tumor cells, MO@NAL efficiently tags the tumor cell surface with OVA through the carried mOVA, providing targets for recruiting and directing the antigen-specific cytotoxic T cells to destroy tumor cells. In mice pre-vaccinated with the OVA vaccine, intratumoral administration of MO@NAL rapidly awakens OVA-specific immune memory, rapidly and effectively inhibiting the progression of colon tumors and melanoma at both early and advanced stages. In non-pre-vaccinated mice, combining MO@NAL with the OVA therapeutic vaccine or OVA-specific adoptive T cell transfusion similarly achieves robust solid tumor suppression. These findings thus underscore the potential of MO@NAL as an effective and safe immunomodulator for enhancing cytotoxic T cell responses and providing timely intervention in solid tumor progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


