高熵单原子间的远程相互作用在催化硫转化反应中的作用
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
硫转化反应是锂硫电池的基础,但在电池实际运行过程中,硫转化反应的动力学通常较慢。为此合成了一种高熵单原子催化剂(HESAC)。与传统的形成金属-金属键的双原子催化剂不同,HESAC的中心金属原子不成键,而是在亚纳米距离上表现出远距离的相互作用(<9 Å)。远程相互作用和熵变之间的协同效应使d-电子态和π-电子态的调控成为可能。这种电子结构的改变改善了中间多硫化物的吸附和电子导电性,从而加快了它们的转化动力学。因此,与单原子催化剂相比,这导致比容量在高速率下显著提高约40%。结果表明,在10℃下,HESAC锂硫电池具有3.4 mAh cm−2的显著面容量。这些发现为电化学反应金属原子催化剂的设计原理提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
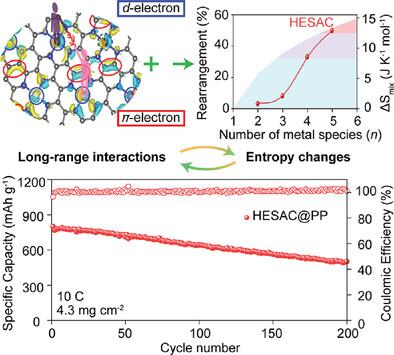
The Role of Long-Range Interactions Between High-Entropy Single-Atoms in Catalyzing Sulfur Conversion Reactions
Sulfur conversion reactions are the foundation of lithium–sulfur batteries but usually possess sluggish kinetics during practical battery operation. Herein, a high-entropy single-atom catalyst (HESAC) is synthesized for this process. In contrast to conventional dual-atom catalysts that form metal–metal bonds, the center metal atoms in HESAC are not bonded but exhibit long-range interactions at a sub-nanometer distance (<9 Å). The synergistic effect between the long-range interactions and entropy changes enables the regulation of d- and π-electron states. This alteration in the electronic structure improves the adsorption and electronic conductivity of intermediate polysulfides, thereby accelerating their conversion kinetics. Consequently, this leads to a significant enhancement in specific capacities by ≈40% at high rates compared to single-atom catalysts. The resulting lithium–sulfur battery with HESAC demonstrates a remarkable areal capacity of 3.4 mAh cm−2 at 10 C. These findings provide valuable insights into the design principle of metal atom catalysts for electrochemical reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


