靶向IL-11纳米颗粒的可吸入siRNA显著抑制博莱霉素诱导的肺纤维化
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对于特发性肺纤维化(IPF),白细胞介素11 (IL-11)是刺激成纤维细胞向肌成纤维细胞转化的关键细胞因子,从而加速肺纤维化的进展。在这里,我们开发了一种名为PEI-GBZA的创新可吸入小干扰RNA (siRNA)递送系统,该系统在装载靶向IL-11的siIL-11 (siIL-11)方面表现出惊人的效率,并显著抑制成纤维细胞向肌成纤维细胞和上皮-间质转化(EMT)的分化,减少中性粒细胞和巨噬细胞的募集,最终缓解IPF模型中已建立的纤维化病变。以4-胍基苯甲酸(GBZA)修饰低分子量聚乙烯亚胺(PEI)制备了PEI-GBZA。由此产生的PEI-GBZA可以通过各种相互作用(如疏水、氢键和静电相互作用)有效地封装siIL-11,形成稳定的载体/siIL-11纳米颗粒(PEI-GBZA/siIL-11 NPs)。吸入后,PEI-GBZA/ sil -11 NPs在纤维化病变中表现出有效的滞留,在博莱霉素诱导的肺纤维化模型中显著减缓疾病进展。令人印象深刻的是,这种吸入疗法的全身毒性可以忽略不计。这项工作为呼吸系统疾病提供了一个通用的、无创的RNA治疗递送平台。该平台的临床应用潜力巨大,为IPF和其他潜在肺部疾病的治疗提供了前沿。本文章由计算机程序翻译,如有差异,请以英文原文为准。
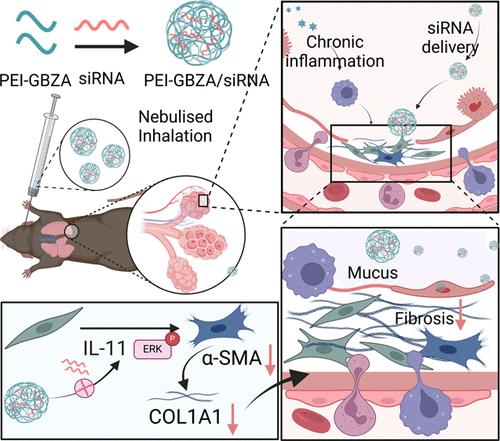
Inhalable siRNA Targeting IL-11 Nanoparticles Significantly Inhibit Bleomycin-Induced Pulmonary Fibrosis
For idiopathic pulmonary fibrosis (IPF), interleukin 11 (IL-11) is a pivotal cytokine that stimulates the transformation of fibroblasts into myofibroblasts, thus accelerating the progression of pulmonary fibrosis. Here, we develop an innovative inhalable small interfering RNA (siRNA) delivery system termed PEI-GBZA, which demonstrates impressive efficiency in loading siIL-11 targeting IL-11 (siIL-11) and substantially suppresses the differentiation of fibroblasts into myofibroblasts and epithelial-mesenchymal transition (EMT), reduces neutrophil and macrophage recruitment, and ultimately relieves the established fibrotic lesions in the IPF model. PEI-GBZA is prepared by modifying low-molecular-weight polyethylenimine (PEI) with 4-guanidinobenzoic acid (GBZA). The resulting PEI-GBZA may effectively encapsulate siIL-11 through a variety of interactions such as hydrophobic, hydrogen bonding, and electrostatic interactions, creating stable carrier/siIL-11 nanoparticles (PEI-GBZA/siIL-11 NPs). Upon inhalation, PEI-GBZA/siIL-11 NPs demonstrate effective retention in fibrotic lesions, leading to a marked mitigation of disease progression in a bleomycin-induced pulmonary fibrosis model. Impressively, this inhalation therapy exhibits negligible systemic toxicity. This work provides a universal and noninvasive RNA therapeutic delivery platform that holds significant promise for respiratory diseases. The potential for clinical application of this platform is substantial, offering a frontier for the treatment of IPF and potentially other pulmonary disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


