四种通过扰乱谷胱甘肽稳态而具有抗肿瘤活性的白耳藤生物碱
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
采用LC/MS直接分离的方法,从Alstoschoquinolines A-D(1-4)中分离到3个史无前例的支架。1和2为5/6/5偶联喹啉结构,包含6个连续的手性碳,3和4为6/6/6/6和6/6/8/6骨架的桥环。它们可能是由堇青素型吲哚生物碱经序贯氧化和重排而来。化合物3通过干扰谷胱甘肽循环对结肠癌细胞表现出明显的抑制作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
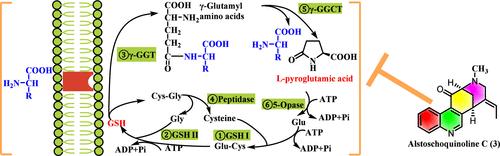
Four Alkaloids from Alstonia scholaris with Antitumor Activity via Disturbing Glutathione Homeostasis
Alstoschoquinolines A–D (1–4) representing three unprecedented scaffolds were isolated from the leaves of Alstonia scholaris through direct separation by LC/MS detection. 1 and 2 consisted of a 5/6/5-coupled quinoline architecture containing six consecutive chiral carbons, while 3 and 4 possessed a bridged ring featuring 6/6/6/6 and 6/6/8/6 skeletons, respectively. They might be derived from the corynantheine-type indole alkaloid via sequential oxidation and rearrangement. Compound 3 exhibited a significant inhibitory effect on colon carcinoma cells by disturbing glutathione circulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


