氢辅助N2解离:负载型Ru催化剂上合成氨的流行及其影响
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
了解共吸附物在拥挤的催化表面上的作用对于优化通常在高压条件下使用的工业催化剂至关重要。对于N2加氢合成氨(即Haber-Bosch法),众所周知负载型Ru催化剂往往被原子H种高度覆盖,而这些H种对N2活化的影响仍存在争议。本文采用动力学评价、同位素标记实验和原位光谱表征相结合的方法,研究了Ru/CeO2催化剂在热处理后结构调整的情况下合成氨的机理。我们的实验方法表明,优势的H*表面物种限制了广泛提出的直接N2解离途径的空位Ru位点的可用性,而是导致H辅助的N2解离途径的流行,其中N2H*中间体中的N-N裂解是一个动力学相关的步骤。根据这一h辅助机制,Ru粒度和Ru - ceo2相互作用对催化活性的影响进行了动力学反旋,揭示了它们分别对本构活性和表面覆盖率的决定性影响。在这些从工作条件中获得的基本见解的驱动下,通过优化Ru颗粒尺寸和金属-载体相互作用,负载型Ru催化剂的氨生成率更高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
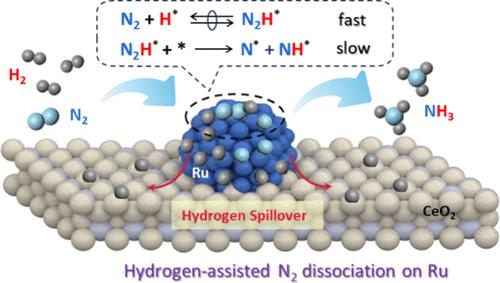
Hydrogen-Assisted Dissociation of N2: Prevalence and Consequences for Ammonia Synthesis on Supported Ru Catalysts
Understanding the roles of coadsorbates on crowded catalytic surfaces is critical to optimizing industrial catalysts that are generally employed under high-pressure conditions. For ammonia synthesis from N2 hydrogenation (i.e., the Haber–Bosch process), it is well-known that supported Ru catalysts tend to be highly covered by atomic H species, while the impact of these H species on N2 activation is still under controversy. Herein, kinetic assessment, isotopic labeling experiments, and in situ spectroscopic characterization were combined to investigate the mechanism of ammonia synthesis on Ru/CeO2 catalysts with their structure tuned via thermal treatments. Our experimental approaches reveal that the dominant H* surface species limit the availability of vacant Ru sites for the widely proposed direct N2 dissociation route but instead lead to the prevalence of the H-assisted N2 dissociation route with the N–N cleavage in N2H* intermediates as a kinetically relevant step. Effects of Ru particle size and Ru–CeO2 interaction on the catalytic activity were kinetically deconvoluted in accordance with this H-assisted mechanism, unveiling their decisive influences on intrinsic activity and surface coverage, respectively. Driven by these fundamental insights gained from the working conditions, superior ammonia formation rates were achieved for supported Ru catalysts via optimizing Ru particle size and metal–support interaction collaboratively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


