原位平衡合成高活性低自旋铁阴极普鲁士蓝以增强钠离子储存
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
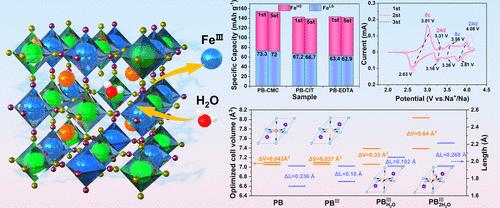
钠离子电池市场的增长激发了对普鲁士蓝阴极材料研究的兴趣。六氰高铁酸铁(FeHCF)被认为是理想的普鲁士蓝型阴极,但其低自旋铁位的电化学性质不完整阻碍了其进一步的实际应用。本文通过DFT计算证明羧甲基纤维素具有合适的结合能,原位合成普鲁士蓝,在FeHCF中平衡Fe3+和水,并引入FeIII空位激活低自旋Fe位点。因此,在1c倍率下,它的初始放电容量为154.7 mAh g-1,能量密度为470.8 Wh kg-1。在100℃下循环4000次后,容量保持率为70.2%。该工作为开发更具成本效益、更快、更耐用的钠离子储能阴极材料提供了一种更简单的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In Situ Balanced Synthesis of High-Activity Low-Spin Iron Cathode Prussian Blue for Enhanced Sodium-Ion Storage
The growing market for sodium-ion batteries has stimulated interest in research on Prussian blue-type cathode materials. Iron hexacyanoferrate (FeHCF) is considered a desirable Prussian blue-type cathode, but the incomplete electrochemical property of its low-spin iron sites hinders its further practical application. In this paper, carboxymethyl cellulose is demonstrated to have an appropriate binding energy through DFT calculations, synthesize Prussian blue in situ, balance Fe3+ and water in FeHCF, and introduce FeIII vacancies to activate low-spin Fe sites. Thus, at a 1 C rate, it achieves an initial discharge capacity of 154.7 mAh g–1 with an energy density of 470.8 Wh kg–1. The capacity retention is 70.2% after 4000 cycles at a rate of 100 C. This work provides a simpler way to develop more cost-effective, faster, and more durable cathode materials for sodium-ion energy storage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


