草甘膦废水在连续反应系统上转化回收为磷肥的过程
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
鉴于磷资源的稀缺和对磷的需求日益增长,对含磷废水中的磷进行循环利用以确保其可持续利用势在必行。传统湿式氧化工艺产生的废水中仍含有相当数量的未充分降解的草甘膦。由于草甘膦的持续存在,这构成了重大的环境风险。在这项工作中,我们通过PMS(过氧单硫酸盐,HSO5−)/氯(Cl-)系统实现了草甘膦废水深度降解成磷酸盐(PO43-)。最后,通过沉淀法从降解废水中回收磷。在草甘膦转化为无机磷的过程中,PMS/Cl-体系产生的活性氯是主要的反应物质(降解贡献率为70.67 ~ 85.5 %),单重态氧(1O2)也是次要的重要氧化途径(降解贡献率为14.5 ~ 29.33 %)。间歇式反应和连续式反应体系均成功地将草甘膦转化为PO43-, PO43-再通过鸟粪石或其他Mg/Ca-P沉淀转化为磷肥,产率为 >; 90 %。从草甘膦废水中回收磷肥不仅是一种技术上可行的工艺,而且具有经济上的优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
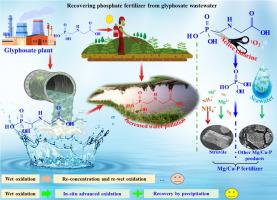
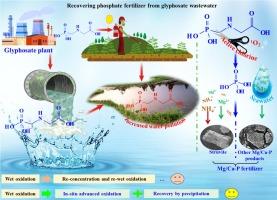
Conversion and recovery process from glyphosate wastewater into phosphatic fertilizer on the continuous reaction system
Given the scarcity of phosphorus resources and the growing demand for phosphorus, it is imperative to recycle phosphorus from wastewater phosphorus-containing to ensure its sustainable use. The wastewater from the conventional wet oxidation process still contains a considerable quantity of glyphosate that has not undergone sufficient degradation. This poses a significant environmental risk due to the persistent presence of glyphosate. In this work, we achieved deep degradation of glyphosate effluent into phosphate (PO43-) by PMS (Peroxymonosulfate, HSO5−)/chlorine (Cl-) system. Finally, phosphorus recovery was achieved by precipitation methods from the degraded effluent. In the conversion of glyphosate to inorganic phosphorus, the reactive chlorine produced by the PMS/Cl- system is the main reactive species (degradation contribution rate of 70.67–85.5 %), and, to a lesser extent, singlet oxygen (1O2) is also an important oxidation pathway (degradation contribution rate of 14.5–29.33 %). Both batch reaction and continuous reaction systems successfully converted glyphosate into PO43-, which was further transformed into phosphatic fertilizer by struvite or other Mg/Ca-P precipitation with > 90 % yield. The recovery of phosphate fertiliser from glyphosate wastewater is not only a technically feasible process, but also economically advantageous.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


