小鼠的命运图谱显示Lgr5+肠干细胞直接从早期分泌分化
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
肠上皮在体内平衡中具有显著的高周转率。这在细胞水平上是如何协调的,以及干细胞和祖细胞的行为如何确保组织的维持,这些问题仍未得到解决。为了解决这个问题,我们将三种互补小鼠模型的定量命运图谱与数学建模和单细胞RNA测序相结合。我们的综合方法生成了一个基于两个循环种群的空间和时间定义的隐窝维持模型:位于隐窝底部的干细胞和位于其上方的转运扩增(TA)细胞。随后,我们验证了数学模型的预测,证明了分泌谱系和吸收谱系之间的命运决定是在干细胞室内做出的,而TA细胞分裂对吸收谱系有专门的贡献。这些定量的见解为隐窝底干细胞作为肠上皮补充的主要驱动因素提供了进一步的直接证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
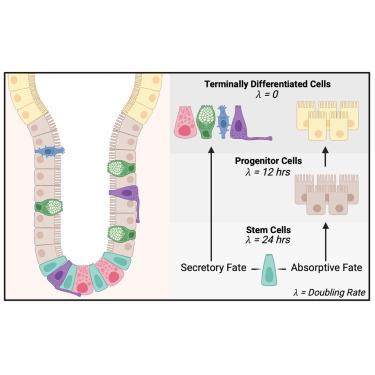
Fate mapping in mouse demonstrates early secretory differentiation directly from Lgr5+ intestinal stem cells
The intestinal epithelium has a remarkably high turnover in homeostasis. It remains unresolved how this is orchestrated at the cellular level and how the behavior of stem and progenitor cells ensures tissue maintenance. To address this, we combined quantitative fate mapping in three complementary mouse models with mathematical modeling and single-cell RNA sequencing. Our integrated approach generated a spatially and temporally defined model of crypt maintenance based on two cycling populations: stem cells at the crypt-bottom and transit-amplifying (TA) cells above them. Subsequently, we validated the predictions from the mathematical model, demonstrating that fate decisions between the secretory and absorptive lineages are made within the stem cell compartment, whereas TA cell divisions contribute specifically to the absorptive lineage. These quantitative insights provide further direct evidence for crypt-bottom stem cells as the dominant driver of the intestinal epithelium replenishment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


