具有丰富极性官能团的酪氨酸添加剂为超稳定锌金属阳极提供了多重保护
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
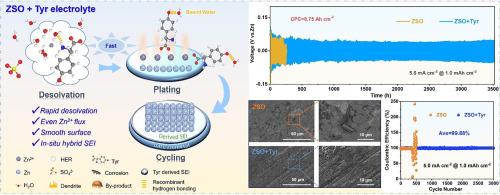
水性锌离子电池(azib)的广泛商业化受到枝晶生长和寄生反应猖獗的严重限制。为了提高锌阳极的稳定性,已经引入了各种添加剂,但单一的极性官能团添加剂并不能对锌阳极提供全面的保护。在这里,一种低成本、高功能的酪氨酸(Tyr)有机小分子被用作电解质添加剂,以实现超稳定的锌金属阳极。结果表明,电负性羧基倾向于参与Zn2+的溶剂化鞘层。亲锌氨基和疏水苯环在锌阳极表面协同形成疏水双电层。羟基的亲水性使其能够捕获自由水分子并重建电解质的氢键网络。更重要的是,Tyr分子的强吸附有利于诱导原位形成有机-无机杂化固体电解质界面层,从而进一步增强对锌阳极的保护作用。得益于Tyr添加剂中多官能团的协同效应,Zn||锌电池在1.0 mA cm - 2,1 mAh cm - 2下表现出超过3800 h的超长循环稳定性(比ZnSO4高出20倍),在5.0 mA cm - 2下具有8.75 Ah cm - 2的超高累积镀容量。此外,经过3000次循环后,Zn||Cu电池的可逆性显著提高,平均库仑效率达到99.88%。这种精细调节的电解质,利用多个官能团的协同效应,预示着持久锌金属电池的发展前景广阔。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Tyrosine additives with rich-polar functional groups provide multi-protections for ultra-stable zinc metal anodes
The widespread commercialization of aqueous Zinc-Ion batteries (AZIBs) is severely limited by dendrite growth and rampant parasitic reactions. While various additives have been introduced to improve the stability of zinc anodes, a single polar functional group additive cannot provide comprehensive protection for zinc anodes. Here, a low-cost, high-functionality Tyrosine (Tyr) organic small molecule is utilized as an electrolyte additive to achieve ultra-stable zinc metal anodes. The findings indicated that the electronegative carboxyl group tended to participate in the solvation sheath of Zn2+. The zinc-philic amino group and hydrophobic benzene ring synergistically construct a hydrophobic electric double layer on the surface of the zinc anode. The hydrophilic nature of the hydroxyl group enables it to capture free water molecules and reconstruct the hydrogen bonding network of the electrolyte. More importantly, the strong adsorption of Tyr molecule is beneficial to induce the formation of an in-situ organic-inorganic hybrid solid electrolyte interface layer, thereby further enhancing protection for the zinc anode. Profiting from the synergistic effect of the polyfunctional group in the Tyr additive, the Zn||Zn cell exhibits ultra-long cycle stability over 3800 h (∼ 20 times vs. ZnSO4) at 1.0 mA cm‒2, 1 mAh cm‒2 and an ultra-high cumulative plated capacity of 8.75 Ah cm‒2 at 5.0 mA cm−2. Furthermore, the Zn||Cu cell delivers a significantly improved reversibility with an average Coulomb efficiency of 99.88 % after 3000 cycles. This finely regulated electrolyte, leveraging the synergistic effects of multiple functional groups, heralds a promising trajectory for the advancement of enduring Zn metal batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


