揭示不同二氧化钛晶相在电催化硝酸还原制氨中的异质界面活化效应
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
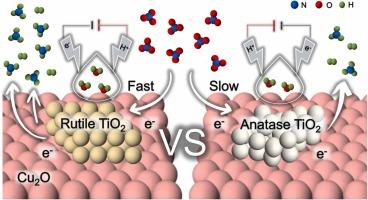
硝酸盐污染对水生生态系统和人类健康构成严重威胁。电催化硝酸还原反应(NITRR)为硝酸盐污染处理和氮源回收提供了一种经济环保的解决方案;然而,它在中性电解质中的效率仍然有限。本研究探讨了金红石(R-TiO2)和锐钛矿(A-TiO2)相TiO2/Cu2O异相催化剂的异质界面活化效应,发现R-TiO2是一种具有高硝酸盐还原性能的活性晶相。在中性电解质中,R-TiO2/Cu2O催化剂在180 min内脱除了99.8%的硝酸盐,氨收率为0.23 mmol h-1 cm-2,法拉第效率为85.7%。原位表征和理论计算表明,异质界面重构和氧空位(OV)的形成克服了R-TiO2电导率差的问题,增强了电子转移,优化了活性位点。此外,界面处的Cu-O-Ti键显著削弱了临界中间体*NO3的吸附能,从而有利于NITRR。本研究为晶体相位调制催化剂的设计提供了新的见解,为开发高效NITRR电催化剂提供了创新策略,为硝酸盐污染的可持续处理和氮源回收铺平了道路。环境影响水生环境中的硝酸盐富集对环境和健康构成重大威胁,需要创新的解决方案。本研究介绍了一种由战略性晶体相位调制驱动的R-TiO2/Cu2O非均相催化剂,该催化剂在NITRR中表现出优异的性能。R-TiO2/Cu2O催化剂在中性电解质中表现出优异的硝酸还原性能,具有快速动力学和高氨选择性的特点。它的高效率和稳定性使其成为有希望通过可持续回收和环境保护来解决硝酸盐污染的下一代催化剂,这对推进清洁水技术至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unveiling heterointerface activation effects with different titanium dioxide crystal phases for electrocatalytic nitrate-to-ammonia reduction
Nitrate pollution poses severe risks to aquatic ecosystems and human health. The electrocatalytic nitrate reduction reaction (NITRR) offers a promising environmental and economic solution for nitrate pollution treatment and nitrogen source recovery; however, it continues to experience limited efficiency in neutral electrolytes. This study explores the heterointerface activation effects of TiO2/Cu2O heterogeneous catalysts with rutile (R-TiO2) and anatase (A-TiO2) phases and reveals that R-TiO2 is an active crystal phase with high nitrate reduction performance. The R-TiO2/Cu2O catalyst removed 99.8 % of nitrate in 180 min and achieved an ammonia yield of 0.23 mmol h−1 cm−2 with a Faraday efficiency of 85.7 % in a neutral electrolyte. In situ characterisation and theoretical calculations revealed that heterointerface reconstruction and oxygen vacancy (OV) formation overcome the poor electrical conductivity of R-TiO2, enhance electron transfer, and optimize the active sites. Furthermore, the Cu-O-Ti bond at the interface significantly weakens the adsorption energy of the critical intermediate *NO3, thereby facilitating NITRR. This study provides new insights into crystal phase modulation in catalyst design and offers innovative strategies for developing highly efficient NITRR electrocatalysts, paving the way for sustainable nitrate pollution treatment and nitrogen source recovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


