LTX-315是一种新型广谱抗多药耐药菌肽
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
由耐多药细菌引起的感染目前对公共卫生构成重大威胁。发现或合成新的化合物是缓解这种紧迫情况的关键。目的验证LTX-315的抑菌活性,探讨其主要作用模式。方法通过抗菌表型试验筛选,从多种药物文库中筛选出1个有效化合物LTX-315,共10,926个化合物。然后,通过时间杀伤曲线、扫描电镜、等温滴定量热分析、核磁共振等生化和化学方法,探讨其杀菌效果及其作用方式。最后,通过小鼠感染模型验证了LTX-315对耐药菌的体内抑制作用。结果本研究发现溶瘤肽LTX-315对革兰氏阳性和革兰氏阴性病原菌均能有效清除,即使对多重耐药菌株也能有效清除。通过强静电相互作用,LTX-315能以极高的亲和力(纳摩尔水平)与膜组分磷脂酰甘油(PG)结合。引人注目的是,与典型的静电相互作用不同,LTX-315的吲哚基团位于烷基链附近,由于烷基链的疏水作用,对PG的识别和相互作用显著增强。此外,它还会对细胞膜产生各种影响,包括破坏细胞膜的完整性、增加细胞膜的通透性和降低细胞膜的流动性。此外,显微镜检查显示明显的细胞解体。这种影响,反过来,破坏细胞内的一些生理活动,如增加活性氧的水平,最终导致细胞死亡。最后,验证了LTX-315在体内对多药耐药和高致病性肺炎克雷伯菌的治疗效果。结论LTX-315与PG的高亲和力结合和随后的膜破坏机制是其独特的机制,与传统抗生素相比,它为治疗多药耐药细菌提供了一种新的途径。作为一种潜在的候选药物,它有望有效治疗细菌感染,特别是由耐药细菌引起的细菌感染,从而解决世界范围内不断升级的抗生素耐药性挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
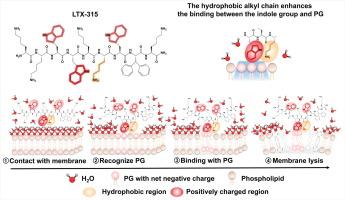
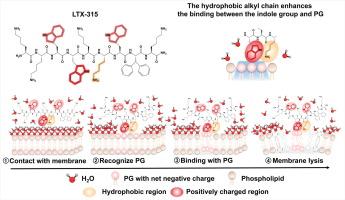
LTX-315 is a novel broad-spectrum antimicrobial peptide against clinical multidrug-resistant bacteria
Introduction
Infections stemming from multidrug-resistant bacteria present a substantial threat to public health today. Discovering or synthesizing novel compounds is crucial to alleviate this pressing situation.
Objective
The main purpose of this study is to verify the antibacterial activity of LTX-315 and explore its primary action mode.
Methods
Through antibacterial phenotype assay screening, we obtained a potent compound named LTX-315 from diverse drug libraries, 10,926 compounds in total. Then, the bactericidal effect and its action mode were explored through biochemical and chemistry methods such as a time-killing curve, scanning electronic microscopy, isothermal titration calorimetry analysis, and nuclear magnetic resonance. Finally, the efficacy in vivo of LTX-315 against drug-resistant bacteria was proved through a mice infection model.
Results
In this study, LTX-315, an oncolytic peptide, was discovered to effectively eliminate gram-positive and gram-negative pathogens, even for those multidrug-resistant strains. Through strong electrostatic interactions, LTX-315 can bind to the membrane component phosphatidylglycerol (PG) with extremely high affinity (nanomolar level). Strikingly, in contrast to the typical electrostatic interactions of antibacterial peptides, the indole group of LTX-315, situated near the alkyl chain, exhibits significantly enhanced recognition and interaction with PG due to the hydrophobic effect of the alkyl chain. Furthermore, it exerts various impacts on cell membranes, including damaging integrity, increasing permeability, and decreasing membrane fluidity. Additionally, microscopy revealed significant cell disintegration. The influence, in turn, disrupts several physiological activities inside cells, such as increasing the reactive oxygen species level, ultimately leading to cell death. Finally, the efficacy of LTX-315 in vivo against multidrug-resistant and hypervirulent Klebsiella pneumoniae was demonstrated.
Conclusion
The unique mechanism of LTX-315 involves high-affinity binding to PG and subsequent membrane disruption, providing a novel approach against multidrug-resistant bacteria compared to conventional antibiotics. As a potential candidate, it shows promise in effectively treating bacterial infections, particularly those caused by drug-resistant bacteria, thereby addressing the escalating challenge of antibiotic resistance worldwide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


