二聚体PD-L1特异性附着体移植铁基纳米片用于肿瘤靶向双模磁共振成像和增强光热免疫治疗
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
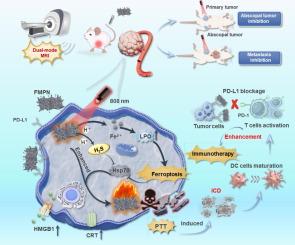
可生物降解的二维纳米材料在生物医学领域的应用引起了人们极大的兴趣。本文研制了一种具有超薄结构和均匀尺寸的可生物降解硫化亚铁纳米片。在其发展的基础上,探索结合Mn-DOTA和二聚体PD-L1粘附体的自增强光热纳米平台(FMPN)在体内的应用,用于靶向双模磁共振成像(MRI)引导的协同肿瘤治疗。采用双对比增强减影成像(DESI)评价FMPN在T1-T2 MRI上的出色表现。FMPN具有良好的光热转换效率(η = 40.33 %)和生物可降解性,具有良好的肿瘤光热治疗效果和良好的生物安全性。此外,ph响应Fe2+释放的FMPN可有效诱导肿瘤细胞铁下垂,进一步加剧PTT和随后的免疫细胞死亡。PD-L1粘附体可以使该纳米平台具有高效的肿瘤靶向性,通过有效阻断PD-1和PD-L1的相互作用,终止T细胞的免疫抑制。在原发性、转移性和腹腔肿瘤中,FMPN的全身递送可显著抑制肿瘤进展,并显示明显的MRI双模增强。因此,该纳米平台在mri引导下的协同铁下垂/光热/免疫治疗中具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dimeric PD-L1 specific affibody grafted Fe-based nanosheets for tumor-targeting dual-mode magnet resonance imaging and enhancement of Photothermal-Immunotherapy
Biodegradable Two-dimensional (2D) nanomaterials have attracted tremendous interest in biomedical application. Herein, a biodegradable ferrous sulfide nanosheet with ultrathin structure and uniform size was developed. Following its development, the application of the self-enhanced photothermal nanoplatform (FMPN) in vivo by combining Mn-DOTA and dimeric PD-L1 affibody for targeted dual-mode magnetic resonance imaging (MRI) guided synergistic tumor therapy is explored. Dual-contrast enhanced subtraction imaging (DESI) was used to evaluate the excellent T1-T2 MRI ability of FMPN. FMPN displayed excellent photothermal conversion efficiency (η = 40.33 %) and biodegradability, thus had good tumor photothermal therapy effects with good biosafety. In addition, FMPN with pH-responsive Fe2+ release can effectively induce tumor cell ferroptosis, further intensifying PTT and subsequent immunologic cell death. PD-L1 affibody could endow this nanoplatform with efficient tumor targeting, terminating T cells’ immune suppression by effectively blocking the interaction between PD-1 and PD-L1. Systemic delivery of FMPN significantly inhibited tumor progression in primary, metastatic and abscopal tumors, and showed obvious dual-mode MRI enhancement. Therefore, this nanoplatform exhibited promising potential for MRI-guided synergistic ferroptosis/photothermal/immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


