阳离子Pd配合物对酮和酯的β-C−H键功能化
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
碳氢活化是有机分子功能化最直接的方法。这一领域的许多进展仍然需要特定的指导组来实现必要的活性和选择性。开发由天然官能团引导的C-H活化反应是其在合成中广泛应用的必要条件。在过去的十年中,开发了几代双功能配体,使游离羧酸2、游离脂肪胺3、天然酰胺4,5和醇6的C(sp3) -H活化反应成为可能。然而,一种有效的酮类和羧酸酯催化剂仍有待开发。在这里,我们报道了多种甲基β-C−H功能化,包括分子间芳基化,羟基化和分子内C(sp3) -H /C(sp2) -H偶联酮和羧酸酯与单保护氨基中性酰胺(MPANA)配体。通过MPANA配体和HBF4的结合原位生成阳离子Pd(II)配合物是实现反应性的关键。这些反应与环酮和内酰胺的相容性为获得螺环和融合环体系提供了一种方法。机制实验和密度泛函理论研究支持阳离子钯配合物与MPANA配体在增强催化剂-底物亲和力和促进C−H裂解步骤中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
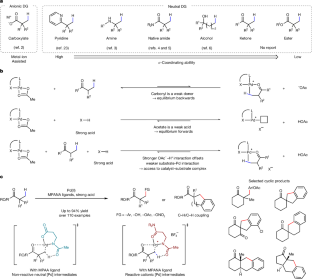

β-C−H bond functionalization of ketones and esters by cationic Pd complexes
C–H activation is the most direct way of functionalizing organic molecules. Many advances in this field still require specific directing groups to achieve the necessary activity and selectivity. Developing C–H activation reactions directed by native functional groups is essential for their broad application in synthesis1. Over the past decade, several generations of bifunctional ligands developed have enabled C(sp3)–H activation reactions of free carboxylic acids2, free aliphatic amines3, native amides4,5 and alcohols6. However, an effective catalyst for ketones and carboxylic esters remains to be realized. Here we report diverse methyl β-C−H functionalizations, including intermolecular arylation, hydroxylation and intramolecular C(sp3)–H/C(sp2)–H coupling of ketones and carboxylic esters with a monoprotected amino neutral amide (MPANA) ligand. The in situ generation of cationic Pd(II) complexes by the combination MPANA ligand and HBF4 is crucial for achieving the reactivity. The compatibility of these reactions with cyclic ketones and lactams provides a method to access spirocyclic and fused ring systems. Mechanistic experiments and density functional theory studies support the role of cationic Pd complexes with MPANA ligands in enhancing catalyst–substrate affinity and facilitating the C−H cleavage step. Diverse methyl β-C−H functionalizations of ketones and esters are enabled by cationic Pd(II) complexes with MPANA ligands, offering access to spirocyclic and fused ring systems through enhanced catalyst–substrate affinity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


