Shethna蛋白对钼氮素酶的构象保护作用
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
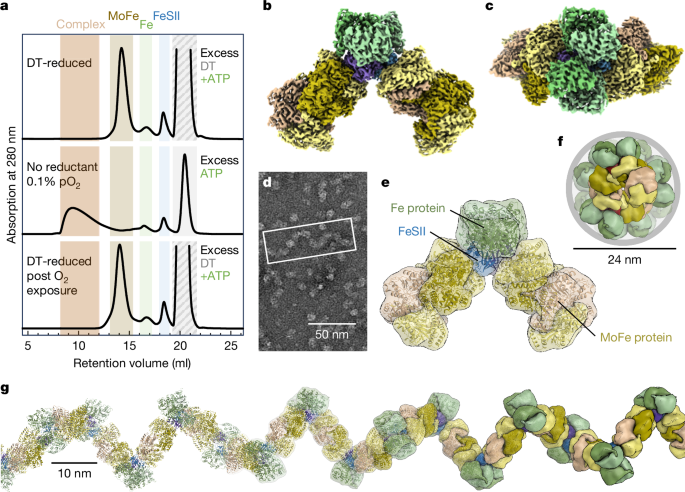
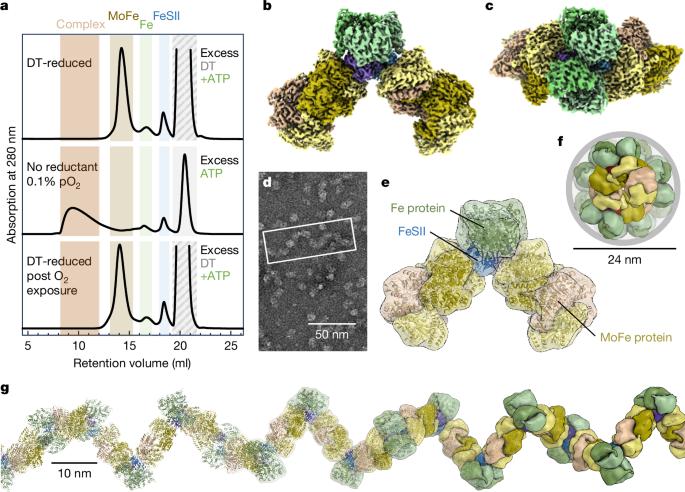
固氮菌的对氧敏感的钼依赖氮酶通过可逆的“关闭”机制免受氧化损伤。它与一个小的铁氧还蛋白FeSII(参考文献2)或“Shethna蛋白II”3形成复合物,当其[2Fe:2S]簇被氧化时,它充当O2传感器并与氮酶的两个组分蛋白结合4,5。本文报道了mo -氮酶催化亚基、其同源还原酶和FeSII蛋白的保护性三元配合物的三维结构,用单粒子冷冻电镜测定。二聚体FeSII蛋白与每个组分的两个拷贝结合,组装一个620 kDa的核心复合物,然后聚合成大的丝状结构。这种复合物没有催化活性,但酶成分在氧气耗尽时迅速释放并重新激活。复合物形成的第一步是FeSII与突发性氧化应激过程中氮酶中对o2更敏感的铁蛋白组分的关联。这种小铁氧还蛋白的作用代表了一种直接的保护O2的手段,这可能是维持粮食作物中重组氮酶的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Conformational protection of molybdenum nitrogenase by Shethna protein II
The oxygen-sensitive molybdenum-dependent nitrogenase of Azotobacter vinelandii is protected from oxidative damage by a reversible ‘switch-off’ mechanism1. It forms a complex with a small ferredoxin, FeSII (ref. 2) or the ‘Shethna protein II’3, which acts as an O2 sensor and associates with the two component proteins of nitrogenase when its [2Fe:2S] cluster becomes oxidized4,5. Here we report the three-dimensional structure of the protective ternary complex of the catalytic subunit of Mo-nitrogenase, its cognate reductase and the FeSII protein, determined by single-particle cryo-electron microscopy. The dimeric FeSII protein associates with two copies of each component to assemble a 620 kDa core complex that then polymerizes into large, filamentous structures. This complex is catalytically inactive, but the enzyme components are quickly released and reactivated upon oxygen depletion. The first step in complex formation is the association of FeSII with the more O2-sensitive Fe protein component of nitrogenase during sudden oxidative stress. The action of this small ferredoxin represents a straightforward means of protection from O2 that may be crucial for the maintenance of recombinant nitrogenase in food crops. A small ferredoxin (Shethna protein II) of Azotobacter vinelandii can provide protection from O2 stress that may be crucial for the maintenance of recombinant nitrogenase in food crops.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


