Cu2O/Al2O3界面中的强电子相互作用使CO2在安培级电流密度下稳定电还原为多碳
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于Cu+的不稳定性、CO2的活化困难以及*CO二聚化缓慢,阻碍了工业安培级CO2RR的实现。本文报道了Cu2O/Al2O3界面诱导的Cu2O纳米空腔中强电子相互作用促进了多碳(C2+)在安培级电流密度下的形成。Cu2O/Al2O3界面偶极子促进了电子从Cu2O向反应物的转移,从而加速了反应动力学,稳定了Cu2O纳米空腔。Al2O3表面较低的电荷密度有利于CO2的活化,通过Al 2p-2π*键的电子反给能削弱*CO中的C-O,促进*CO的二聚化。同时,这些Al2O3层可以调节局部微环境,进一步激活CO2,促进*CO二聚化。因此,该催化剂在1200 mA cm−2时具有84.7%的C2+法拉第效率。在1600 mA cm−2时,C2+的生成速率和能量转换效率分别达到3.7 mmol h−1 cm−2和34.0%。此外,该催化剂在100 cm2的膜电极组件(MEA)电解槽中显示出工业电流(30 A)的电解能力,并具有显着的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
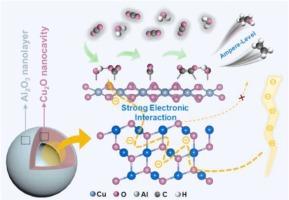
Strong electronic interaction in Cu2O/Al2O3 interface for stable electroreduction of CO2 to multi-carbon with ampere-level current density
Achieving industrial ampere-level CO2RR is hindered due to the instability of Cu+ species, the difficulty in CO2 activation, and the sluggish *CO dimerization. Herein, the strong electronic interaction in Cu2O nanocavities induced by Cu2O/Al2O3 interface is reported to promote multi-carbon (C2+) formation at ampere-level current density. The Cu2O/Al2O3 interfacial dipoles promote the electrons transfer from Cu2O to reactants, thereby accelerating the reaction kinetics and stabilizing the Cu2O nanocavities. The lower charge density on the Al2O3 surface facilitate CO2 activation, and the electron backdonation via Al 2p-2π* bond weaken the C-O in *CO, promoting *CO dimerization. Meanwhile, such Al2O3 layers can modulate the local microenvironment to further activate CO2 and promote *CO dimerization. Hence, the catalyst exhibits C2+ Faradaic efficiency of 84.7 % at 1200 mA cm−2. The formation rate and the energy conversion efficiency of C2+ reaches 3.7 mmol h−1 cm−2 and 34.0 % at 1600 mA cm−2, respectively. Moreover, the catalyst demonstrates an industrial-current (30 A) electrolysis capability with remarkable stability in a 100 cm2 membrane electrode assembly (MEA) electrolyzer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


