MDM2作为一个计时器,报告有丝分裂的长度
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
有丝分裂的延迟会在下一个细胞周期的G1期触发p53依赖性阻滞,从而防止染色体不稳定和非整倍体的重复周期。我们发现MDM2,即p53泛素连接酶,是有丝分裂延长时触发G1阻滞的计时器机制的关键组成部分。这种计时器功能是由于有丝分裂中蛋白质合成的衰减而产生的。由于MDM2的半衰期短,因此需要持续的蛋白质合成来维持其稳态浓度,MDM2的量在有丝分裂期间逐渐下降,但在G1开始时通常保持在p53调节的临界阈值以上。当纺锤体组装检查点激活延长有丝分裂时,MDM2的数量下降到这个阈值以下,从而稳定p53。随后p53依赖性p21的积累引导G1细胞进入持续的细胞周期阻滞,而p53缺陷细胞的应答消除使它们绕过这一关键的防御机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

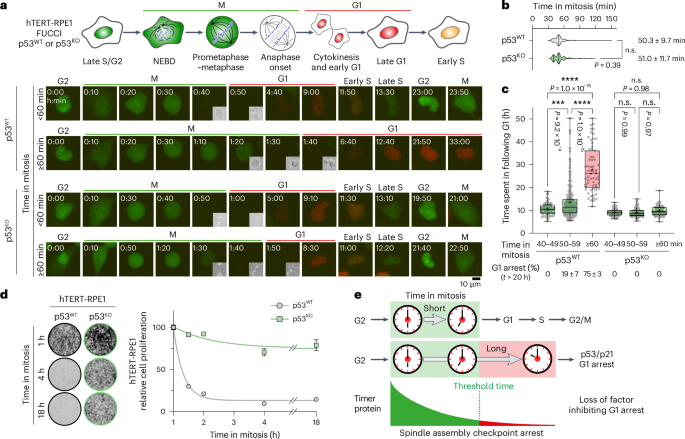
MDM2 functions as a timer reporting the length of mitosis
Delays in mitosis trigger p53-dependent arrest in G1 of the next cell cycle, thus preventing repeated cycles of chromosome instability and aneuploidy. Here we show that MDM2, the p53 ubiquitin ligase, is a key component of the timer mechanism triggering G1 arrest in response to prolonged mitosis. This timer function arises due to the attenuation of protein synthesis in mitosis. Because MDM2 has a short half-life and ongoing protein synthesis is therefore necessary to maintain its steady-state concentration, the amount of MDM2 gradually falls during mitosis but normally remains above a critical threshold for p53 regulation at the onset of G1. When mitosis is extended by prolonged spindle assembly checkpoint activation, the amount of MDM2 drops below this threshold, stabilizing p53. Subsequent p53-dependent p21 accumulation then channels G1 cells into a sustained cell-cycle arrest, whereas abrogation of the response in p53-deficient cells allows them to bypass this crucial defence mechanism. Fulcher et al. show that MDM2 times mitosis through self-catalysed ubiquitination and proteasomal destruction, triggering G1 arrest following delays in mitosis associated with chromosome instability and aneuploidy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


