V2O5/g-C3N4异质结增强过氧单硫酸盐活化对氯霉素的快速降解:内置电场的作用
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
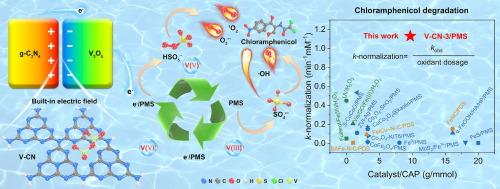
与传统金属氧化物只进行两价态转换相比,多价金属氧化物提供了额外的电子,从而加速了过氧单硫酸盐(PMS)的活化,提高了催化活性。然而,高价和低价金属离子之间的低转换效率往往限制了催化性能。在这项研究中,我们采用水热聚合和热聚合相结合的策略将多价五氧化二钒(V2O5)负载到石墨氮化碳(g-C3N4)上,从而构建了V2O5/g-C3N4异质结。该异质结形成了一个内置电场,促进了PMS的激活,并以1.13 min−1的速率常数实现了破纪录的氯霉素(CAP)降解。实验和理论分析表明,自由基和非自由基途径之间的协同作用是有效去除CAP的主要机制。内置电场改变了轨道占用,减小了带隙,增强了电子从g-C3N4到V2O5的转移,加速了钒价态之间的转换,增强了PMS活化。此外,V2O5/g-C3N4/PMS体系表现出较宽的工作pH范围(2-10),对基质干扰的抵抗能力强,以及优异的耐久性,证明了其在减轻CAP生态毒性和处理典型难降解有机污染物方面的适用性。这项研究推进了对内置电场如何促进PMS激活的理解,并为解决水生环境中新出现的污染物引入了一种新的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced peroxymonosulfate activation by V2O5/g-C3N4 heterojunction for rapid degradation of chloramphenicol: Role of the built-in electric field
In contrast to traditional metal oxides that engage in only two valence state conversions, multivalent metal oxides offer additional electrons, thus accelerating the activation of peroxymonosulfate (PMS) and enhancing catalytic activity. However, the low efficiency of converting between high-valent and low-valent metal ions often limits catalytic performance. In this study, we employed a combined hydrothermal and thermal polymerization strategy to load multivalent vanadium pentoxide (V2O5) onto graphitic carbon nitride (g-C3N4), thereby constructing a V2O5/g-C3N4 heterojunction. This heterojunction formed a built-in electric field, facilitating PMS activation and achieving a record-breaking degradation of chloramphenicol (CAP) with a rate constant of 1.13 min−1. Experimental and theoretical analyses indicated that the synergy between radical and non-radical pathways was the primary mechanism for efficient CAP removal. The built-in electric field altered orbital occupancy, reduced the bandgap, and enhanced electron transfer from g-C3N4 to V2O5, accelerating the conversion between vanadium valence states and enhancing PMS activation. Furthermore, the V2O5/g-C3N4/PMS system exhibited a broad operational pH range (2–10), robust resistance to matrix interference, and exceptional durability, demonstrating its applicability for mitigating ecological toxicity of CAP and treating typical refractory organic pollutants. This study advances the understanding of how built-in electric fields promote PMS activation and introduces a novel approach for addressing emerging pollutants in aquatic environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


