远距离金属-吸附剂相互作用决定CO2在双功能材料中的捕获和转化
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
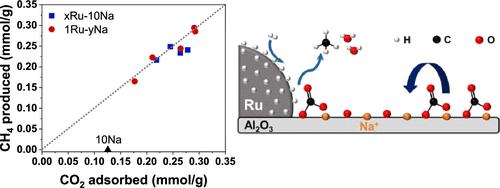
碳捕获和利用涉及多个能源和成本密集型步骤。双功能材料(dfm)可以通过将二氧化碳吸附和转化耦合成具有两种功能的单一材料(吸附剂相和用于催化二氧化碳转化的金属)来减少这些需求。从以往的工作来看,金属催化剂在转化过程中的作用似乎是显著的,但其潜在的机制仍然难以捉摸,需要更深入的研究,以实现两相的最大利用。在这里,预先形成的胶体Ru纳米颗粒沉积在“NaOx”/Al2O3吸附剂上,以制备具有控制相的原型dfs,用于CO2捕获和氢化成CH4。在还原预处理阶段,Ru的加入通过激活NaOx /Al2O3吸附剂相,使高温CO2吸附能力提高了一倍。最重要的是,低Ru负载足以确保最大的CO2吸附和转化。这归因于金属-吸附剂相互作用的关键作用,其中Ru需要通过Ru上活化的H2将“NaOx”/Al2O3吸附剂上的强结合CO2氢化成CH4。这种相互作用促进了决定速率的碳酸盐迁移和随后在金属-吸附剂界面上的氢化。总体而言,Ru控制CO2加氢反应速率,而NaOx /Al2O3吸附剂控制CO2吸收能力。通过在分子水平上控制金属-吸附剂的相互作用,我们证明了两相及其协同作用的关键作用,促进了具有最大二氧化碳捕获和转化效率的DFMs的设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Long-Range Metal–Sorbent Interactions Determine CO2 Capture and Conversion in Dual-Function Materials
Carbon capture and utilization involve multiple energy- and cost-intensive steps. Dual-function materials (DFMs) can reduce these demands by coupling CO2 adsorption and conversion into a single material with two functionalities: a sorbent phase and a metal for catalytic CO2 conversion. The role of metal catalysts in the conversion process seems salient from previous work, but the underlying mechanisms remain elusive and deserve deeper investigation to achieve maximum utilization of the two phases. Here, preformed colloidal Ru nanoparticles were deposited onto a “NaOx”/Al2O3 sorbent to prepare prototypical DFMs with controlled phases for CO2 capture and hydrogenation to CH4. Ru addition was found to double the high-temperature CO2 adsorption capacity by activating the “NaOx”/Al2O3 sorbent phase during a reductive pretreatment step. Most importantly, low Ru loadings were sufficient to ensure maximum CO2 adsorption and conversion. This was attributed to the key role of the metal–sorbent interactions, wherein Ru was required to hydrogenate strongly bound CO2 on the “NaOx”/Al2O3 sorbent to CH4 via the H2 activated on Ru. This interaction facilitated rate-determining carbonate migration and subsequent hydrogenation at the metal–sorbent interface. Overall, Ru controlled the CO2 hydrogenation reaction rate, while the “NaOx”/Al2O3 sorbent dictated the CO2 uptake capacity. By controlling metal–sorbent interactions at the molecular level, we demonstrate the critical role of the two phases and their synergy, facilitating the design of DFMs with maximum CO2 capture and conversion efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


