高压水锌-碘电池中I+的尿素螯合作用
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
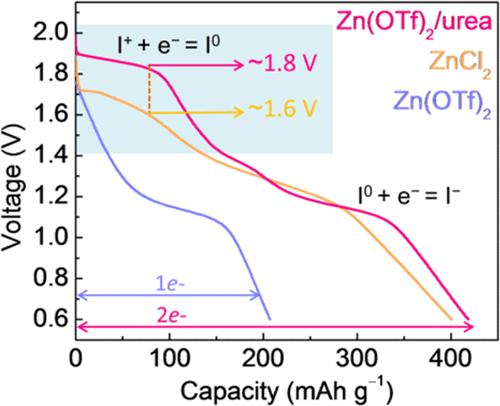
通过I - /I0/I+的多电子转换电化学,实现了锌碘电池的高比容量和高电压。不幸的是,I+离子在热力学上是不稳定的,很容易被水解。目前的工作主要集中在探索卤素间化学来激活I0/I+偶联。然而,实际工作电压低于理论水平。在本研究中,I0/I+氧化还原对被充分激活,I+在常规的水电解质中被高效的尿素螯合剂稳定。实现了创纪录的1.8 V vs Zn/Zn2+平台电压。理论计算结合光谱研究和电化学测试表明,尿素分子中缺电子的I+与富电子的O和N原子之间的配位在热力学上有利于I0/I+的转化,抑制了I+的自歧化,从而促进了I0/I+的快速动力学和良好的可逆性。此外,尿素通过形成氢键来降低电解质中的水活度,从而进一步抑制I+的水解。因此,在1C下提供419 mAh g-1的高比容量,在5C下循环10,000次后保持147 mAh g-1容量。这项工作为配制高性能水锌碘电池的无卤电解质提供了有效的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Urea Chelation of I+ for High-Voltage Aqueous Zinc–Iodine Batteries
The multielectron conversion electrochemistry of I–/I0/I+ enables high specific capacity and voltage in zinc–iodine batteries. Unfortunately, the I+ ions are thermodynamically unstable and are highly susceptible to hydrolysis. Current endeavors primarily focus on exploring interhalogen chemistry to activate the I0/I+ couple. However, the practical working voltage is below the theoretical level. In this study, the I0/I+ redox couple is fully activated, and I+ is efficiently stabilized by a chelation agent of cost-effective urea in the conventional aqueous electrolyte. A record-high plateau voltage of 1.8 V vs Zn/Zn2+ has been realized. Theoretical calculations combined with spectroscopy studies and electrochemical tests reveal that the coordination between the electron-deficient I+ and the electron-rich O and N atoms in urea molecules is thermodynamically favorable for I0/I+ conversion and inhibits the self-disproportionation of I+, which in turn promotes rapid kinetics and excellent reversibility of I0/I+. Moreover, urea decreases the water activity in the electrolyte by forming hydrogen bonds to further suppress the hydrolysis of I+. Accordingly, a high specific capacity of 419 mAh g–1 is delivered at 1C, and 147 mAh g–1 capacity is retained after 10,000 cycles at 5C. This work offers effective insights into formulating halogen-free electrolytes for high-performance aqueous zinc–iodine batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


