位和对映选择性烯丙基和丙炔C-H氧化由铜基仿生催化实现
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
直接对映选择性氧化C(sp3) -H键的方法将给手性醇及其衍生物的制备带来革命性的变化。酶催化是一种利用关键的金属氧物种来促进有效的氢原子提取的方法,已经发展成为生物系统中C-H氧化的一种高选择性方法。尽管它是有效的,但在仿生催化剂中再现这种功能并实现高立体选择性已被证明是一项艰巨的任务。在这里,我们提出了一个基于铜的仿生催化系统,以C-H底物作为限制试剂,实现了高效的不对称sp3 C-H氧化。Cu(II)结合的叔丁基自由基负责位点选择性的C-H键切割,类似于铜基酶的C-H氧化活性位点。该方法具有良好的官能团相容性和极高的位点选择性和对映体选择性,适用于生物活性化合物的后期氧化。本文章由计算机程序翻译,如有差异,请以英文原文为准。

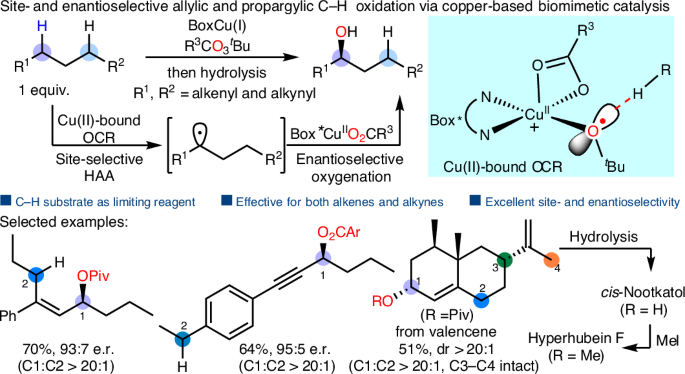
Site- and enantioselective allylic and propargylic C–H oxidation enabled by copper-based biomimetic catalysis
Methods for direct enantioselective oxidation of C(sp3)–H bonds will revolutionize the preparation of chiral alcohols and their derivatives. Enzymatic catalysis, which uses key metal-oxo species to facilitate efficient hydrogen atom abstraction, has evolved as a highly selective approach for C–H oxidation in biological systems. Despite its effectiveness, reproducing this function and achieving high stereoselectivity in biomimetic catalysts has proven to be a daunting task. Here we present a copper-based biomimetic catalytic system that achieves highly efficient asymmetric sp3 C–H oxidation with C–H substrates as the limiting reagent. A Cu(II)-bound tert-butoxy radical is responsible for the site-selective C–H bond cleavage, which resembles the active site of copper-based enzymes for C–H oxidation. The developed method has been successfully accomplished with good functional group compatibility and exceptionally high site- and enantioselectivity, which is applicable for the late-stage oxidation of bioactive compounds. The efficiency of enantioselective sp3 C–H bond oxidation using small synthetic catalysts is usually limited. Now a catalytic system involving a Cu(II)-bound tert-butoxy radical for site-selective C–H bond cleavage achieves allylic and propargylic sp3 C–H oxidation with the C–H substrates as the limiting reagent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


