将转基因猪心脏移植到活人体内
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
根据我们之前将转基因猪心脏移植到活人体内的经验,我们试图通过选择更健康的受体和对潜在的人畜共患病病原体进行更敏感的供体筛选来获得更好的结果。在这里,我们将一颗10基因编辑的猪心脏移植到一名58岁的男性患者身上,该患者因缺血性心肌病而患有进行性、衰弱性肌力依赖性心力衰竭,他不是标准晚期心力衰竭治疗的候选者。患者维持共刺激(抗cd40l, tegopruart)阻断免疫调节方案。异种移植物最初功能良好,在移植后的最初几周内具有良好的收缩和舒张功能。随后,异种移植物迅速发展为舒张性心力衰竭,双心室壁增厚,最终几乎完全丧失收缩功能,需要在第31天开始体外膜氧合。考虑到这些挫折,患者选择在40天后过渡到舒适护理。与我们的第一位患者一样,组织学未显示大量免疫细胞浸润,但提示毛细血管内皮损伤伴间质水肿和早期纤维化。在异种移植物中没有观察到猪巨细胞病毒复制的证据。为了推进异种移植领域的发展,需要克服抗体介导的排斥障碍的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。

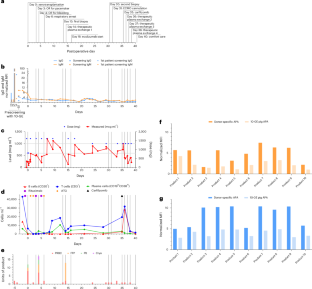
Transplantation of a genetically modified porcine heart into a live human
Following our previous experience with cardiac xenotransplantation of a genetically modified porcine heart into a live human, we sought to achieve improved results by selecting a healthier recipient and through more sensitive donor screening for potential zoonotic pathogens. Here we transplanted a 10-gene-edited pig heart into a 58-year-old man with progressive, debilitating inotrope-dependent heart failure due to ischemic cardiomyopathy who was not a candidate for standard advanced heart failure therapies. He was maintained on a costimulation (anti-CD40L, Tegoprubart) blockade-based immunomodulatory regimen. The xenograft initially functioned well, with excellent systolic and diastolic function during the first several weeks posttransplantation. Subsequently, the xenograft developed rapidly progressing diastolic heart failure, biventricular wall thickening and, ultimately, near-complete loss of systolic function necessitating initiation of extracorporeal membranous oxygenation on day 31. Given these setbacks, the patient chose to transition to comfort care after 40 days. As with our first patient, histology did not reveal substantial immune cell infiltration but suggested capillary endothelial injury with interstitial edema and early fibrosis. No evidence of porcine cytomegalovirus replication in the xenograft was observed. Strategies to overcome the obstacle of antibody-mediated rejection are needed to advance the field of xenotransplantation. In the second case in which a genetically modified pig heart was transplanted into a living person, the xenografted heart functioned well initially, but antibody-mediated rejection occurred thereafter, pointing to the need for improved strategies to avoid this complication.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


