含芳基和氰基双取代ε-己内酯的合成及开环聚合
IF 4.5
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
以市售芳基腈和丙烯酸甲酯为原料,设计并合成了一系列新的芳基和氰基相连接的4,4-二取代ε-己内酯(CL)单体。合成过程依赖于串联双Michael加成- dieckmann缩合- krapcho脱羧在一锅体系中,然后是随后的bayer - villiger氧化。此外,在温和的反应条件下,以对甲基苄基醇为引发剂,1,5,7-三氮杂环[4.4.0]十二-5-烯(TBD)为有机催化剂,成功地实现了这些氯单体的开环聚合。所得聚酯的玻璃化转变温度(Tg)在45 ~ 95℃之间,强烈依赖于氰基和芳基取代基。更重要的是,合成的聚酯可以在碱条件下有效解聚,生成相应的ε-羟基己酸,产率高,并可通过后续内酯化过程转化回起始单体,从而建立循环生命周期。本文章由计算机程序翻译,如有差异,请以英文原文为准。
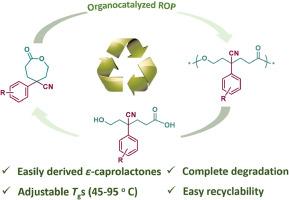

Synthesis and ring-opening polymerization of bisubstituted ε-caprolactones bearing aryl and cyano groups
A series of novel 4,4-bisubstituted ε-caprolactone (CL) monomers with pendent aryl and cyano groups were designed and effectively synthesized from commercially available aryl nitriles with methyl acrylate. The synthetic process relies on a tandem double Michael addition-Dieckmann condensation-Krapcho decarboxylation in a one-pot system, followed by a subsequent Baeyer-Villiger oxidation. Furthermore, the ring-opening polymerization of these obtained CL monomers was successfully achieved under mild reaction conditions, employing p-methylbenzyl alcohol as the initiator and 1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) as an organocatalyst. The glass transition temperatures (Tg) of the resultant polyesters were in the range of 45–95 °C, strongly dependent on the cyano and aryl substituents. More importantly, the resultant polyesters could be effectively depolymerized in alkali condition to generate the corresponding ε-hydroxy caproic acids with excellent yields, which could be transformed back to their starting monomers via subsequent lactonization process, thus establishing their circular life cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


