Cr-Cu2O表面加速电解促进苯乙炔电催化加氢
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
以水为氢源的电化学炔还原是一条可持续的高附加值烯烃生产途径。但Cu电极吸附氢(*H)生成能力弱,导致其电压高、活性低,影响了反应效率。在这篇文章中,我们提出了苯乙炔的增强电催化苯乙烯在一个高分散的cr掺杂Cu2O纳米线(Cr-Cu2O)阴极。与纯Cu2O相比,Cr-Cu2O具有更高的催化活性,在−1.15 V vs Hg/HgO的低电位下,转化率高达94.7%,选择性为87.9%,法拉第效率为64.5%。电化学表征技术与理论计算相结合,证明了引入Cr原子在降低地表水电解至*H的活化能势和促进苯乙炔吸附方面的关键作用,从而通过电催化加氢机制促进苯乙炔与*H的有效加氢。总之,本工作提供了一种可行的富集界面*H的策略,从而提高了苯乙炔的半加氢性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
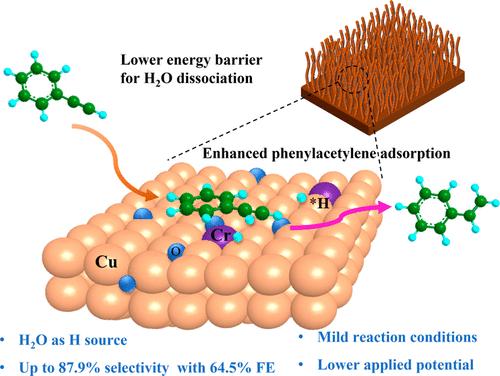
Boosting Electrocatalytic Hydrogenation of Phenylacetylene via Accelerating Water Electrolysis on a Cr-Cu2O Surface
Electrochemical alkyne reduction with H2O as a hydrogen source represents a sustainable route for value-added olefin production. However, the reaction efficiency is hampered by the high voltage and low activity of Cu electrodes due to their weak adsorbed hydrogen (*H) generation property. In this article, we present the enhanced electrocatalysis of phenylacetylene to styrene over a highly dispersive Cr-doped Cu2O nanowire (Cr-Cu2O) cathode. The Cr-Cu2O demonstrates improved catalytic activity compared to pure Cu2O, achieving a high conversion of about 94.7% and a selectivity of 87.9% with a Faraday efficiency of 64.5% at a low potential of −1.15 V vs Hg/HgO. The combination of electrochemical characterization techniques and theoretical calculations demonstrated the key role of introduced Cr atoms in lowering the activation energy barrier of surface water electrolysis to *H and facilitating the adsorption of phenylacetylene, which promotes the effective hydrogenation of phenylacetylene with *H via an electrocatalytic hydrogenation mechanism. In short, this work provides a feasible strategy to enrich interfacial *H, thus improving the semihydrogenation performance of phenylacetylene.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


