动态力量决定了被淘汰细胞的生存命运
IF 17.6
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
摘要
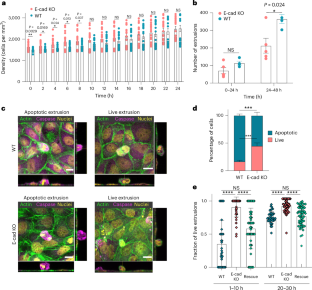
组织通过细胞挤压消除不合适的、不需要的或不必要的细胞,这可以导致凋亡细胞和活细胞的消除。然而,影响挤压细胞命运的机械特征仍然未知。在这里,我们表明,修改的力传递通过粘附连接抑制凋亡细胞的消除。通过结合不同水平e-钙粘蛋白连接的细胞实验和细胞单层的三维建模,我们发现这些变化不仅影响挤压细胞的命运,而且使挤压从根尖向基底侧转移,导致细胞侵入软胶原凝胶。我们使用来自乳腺癌患者的异种移植物和基质培养的囊肿来推广我们的发现。我们的研究结果将细胞间力传递与细胞挤压机制联系起来,这在癌细胞的形态发生和侵袭过程中具有潜在的意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Dynamic forces shape the survival fate of eliminated cells
Tissues eliminate unfit, unwanted or unnecessary cells through cell extrusion, and this can lead to the elimination of both apoptotic and live cells. However, the mechanical signatures that influence the fate of extruding cells remain unknown. Here we show that modified force transmission across adherens junctions inhibits apoptotic cell eliminations. By combining cell experiments with varying levels of E-cadherin junctions and three-dimensional modelling of cell monolayers, we find that these changes not only affect the fate of the extruded cells but also shift extrusion from the apical to the basal side, leading to cell invasion into soft collagen gels. We generalize our findings using xenografts and cysts cultured in matrigel, derived from patients with breast cancer. Our results link intercellular force transmission regulated by cell–cell communication to cell extrusion mechanisms, with potential implications during morphogenesis and invasion of cancer cells. Tissues eliminate unwanted cells through cell extrusion, but the factors determining whether these extuded cells live or die are not fully understood. Now force transmission across adherens junctions is shown to have a role in shaping their fate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


