用Pd簇修饰Cu纳米粒子,通过有效氢溢出增强腈电加氢制伯胺
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
苯腈的氢参与电化学加氢(ECH)是一种温和而高效的合成苯胺的方法,但其动力学和法拉第效率仍然有限。本文将钯簇分散的Cu纳米颗粒包埋在多孔碳(Pdn-Cu@C)中,实现了苯腈(C6H5CN)与苄胺(C6H5CH2NH2)的高效ECH反应。原位红外光谱和理论研究表明,Pd/Cu界面作为H2O解离生成的活性氢(*H)的活性位点,增强了对C6H5CN的吸附,减弱了对C6H5CH2NH2的吸附。此外,*H溢出的吉布斯自由能垒远低于*H自耦合的吉布斯自由能垒。结果表明,Pdn-Cu@C在特定电压下,C6H5CN的电加氢转化率为97.42%,C6H5CH2NH2选择性为97.21%,法拉第效率为92.10%。这一发现为抑制竞争*H自偶联开辟了一条可行的途径,并为多步质子化ECH反应提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
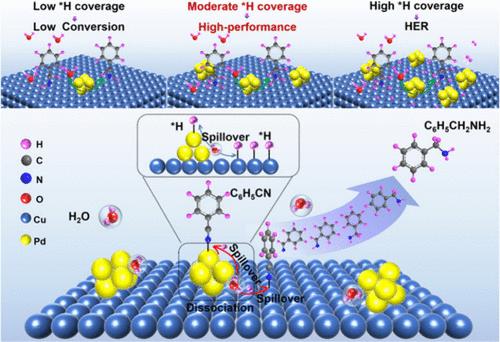
Decorating Cu Nanoparticles with Pd Clusters for Enhanced Nitrile Electro-Hydrogenation to Primary Amines by Effective Hydrogen Spillover
The H2O-participating electrochemical hydrogenation (ECH) of benzonitrile represents a mild and efficient method for benzylamine synthesis, but the kinetics and Faraday efficiency are still limited. Herein, the developed Pd clusters dispersed Cu nanoparticles encapsulated in porous carbon (Pdn-Cu@C) achieves efficient ECH of benzonitrile (C6H5CN) to benzylamines (C6H5CH2NH2). In situ infrared spectroscopy and theoretical studies reveal that the Pd/Cu interface functions as the active site for active hydrogen (*H) generated by H2O dissociation, enhances the adsorption of C6H5CN, and weakens the adsorption of C6H5CH2NH2. Moreover, the Gibbs free energy barriers for *H spillover are much lower than that of *H self-coupling. As expected, Pdn-Cu@C exhibits efficient electro-hydrogenation of C6H5CN with the conversion of 97.42%, a high C6H5CH2NH2 selectivity of 97.21%, and Faradaic efficiency of 92.10% under a specific voltage. This finding blazes a feasible trail to suppress the competitive *H self-coupling and offers insights for multistep protonation ECH reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


