微量SO2对胺吸收剂对CO2化学吸收的影响
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
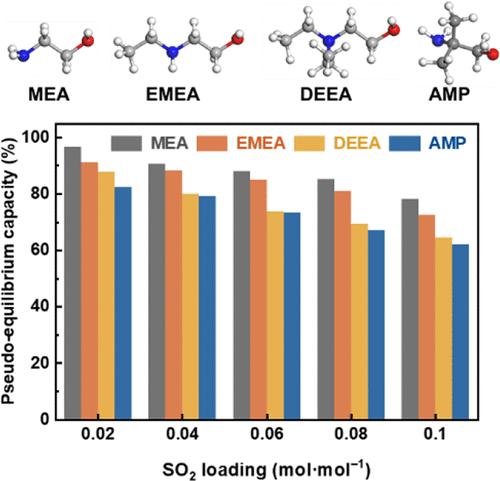
工业烟气中微量SO2对CO2过程中胺基化学吸收的影响是一个值得关注的问题。本文研究了四种代表性的胺类吸附剂对痕量二氧化硫的影响,这些吸附剂含有乙醇胺(MEA)的伯胺基、2-乙胺乙醇(EMEA)的仲胺基、N,N-二乙基乙醇胺(DEEA)的叔胺基和2-氨基-2-甲基-1-丙醇(AMP)的位阻胺基,重点研究了它们的吸收/解吸能力、循环吸收性能、CO2/SO2选择性、抗SO2能力。实验结果表明,具有伯胺基的MEA吸胺剂具有最高的抗SO2能力,而具有叔胺基的EMEA吸胺剂具有最低的抗SO2能力。此外,CO2/SO2选择性研究表明,含有伯胺基团的MEA吸附剂对CO2的选择性优于其他吸附剂。此外,核磁共振分析显示,与氨基甲酸酯形成相比,SO2对碳酸氢盐形成的抑制作用更显著,这表明吸收CO2后容易形成氨基甲酸酯的胺类吸收物具有更强的抗SO2能力。总之,这项工作为利用胺吸收剂在SO2存在下捕获CO2提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Insights into the Impact of Trace SO2 on Amine Absorbents for Chemical Absorption of CO2
The impact of trace SO2 in industrial flue gas on the amine-based chemical absorption of the CO2 process is a significant concern. This work investigates the impact of trace SO2 on the chemical absorption of CO2 by four representative amine absorbents containing a primary amine group of ethanolamine (MEA), a secondary amine group of 2-(ethylamino)ethanol (EMEA), a tertiary amine group of N,N-diethylethanolamine (DEEA), and a sterically hindered amine group of 2-amino-2-methyl-1-propanol (AMP), focusing on their absorption/desorption capacity, cyclic absorption performance, CO2/SO2 selectivity, and SO2 resistance capability. Experimental results revealed that the amine absorbent of MEA with the primary amine group has the highest SO2 resistance capability, while the amine absorbent of EMEA with the tertiary amine group exhibits the lowest. In addition, CO2/SO2 selectivity investigations indicated that the amine absorbent of MEA with the primary amine group has superior selectivity for CO2 than others. Moreover, NMR analysis revealed that SO2 more significantly inhibited bicarbonate formation compared to carbamate formation, suggesting that amine absorbents prone to carbamate formation after CO2 absorption have greater SO2 resistant capability. Altogether, this work provides valuable insight into the utilization of amine absorbents for the capture of CO2 in the presence of SO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


