用混合硝酰胺炸药研究含能材料的触发连锁动力学
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
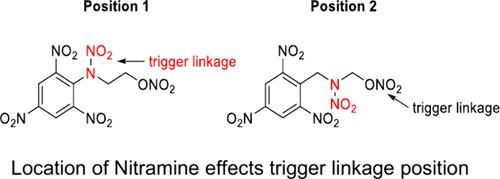
预测新型有机含能材料的处理灵敏度一直是一个长期的目标。我们报道了六种新的硝基酰胺含能材料的合成和表征,这些材料具有混合官能团,可以模拟已知的炸药,如硝酸甘油、四硝酸赤藓糖醇(ETN)和四硝酸季戊糖醇(PETN)。利用量子分子动力学(QMD)模拟和密度泛函理论(DFT)计算对分子进行了理论研究,以确定反应物中最弱的键-触发键-控制处理灵敏度,并量化它们的特定爆炸焓。与跌落重量冲击灵敏度数据一致,我们的计算预测,由于能量输出和触发连杆断裂率的微小变化,分子的灵敏度非常相似。此外,QMD和DFT计算都指出硝基酰胺的N-NO2键是触发键,而不是更典型的O-NO2键。我们认为触发键从硝酸酯到硝胺基团的转换是由于相邻三硝基苯基团的强吸电子特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Understanding Trigger Linkage Dynamics in Energetic Materials Using Mixed Picramide Nitrate Ester Explosives
The ability to predict the handling sensitivity of new organic energetic materials has been a longstanding goal. We report the synthesis and characterization of six new nitropicramide energetic materials with mixed functional groups that mimic known explosives such as nitroglycerin, erythritol tetranitrate (ETN), and pentaerythritol tetranitrate (PETN). The molecules have been studied theoretically using quantum molecular dynamics (QMD) simulations and density functional theory (DFT) calculations to identify the weakest bond in the reactants - the trigger-linkages - which control handling sensitivity, and to quantify their specific enthalpies of explosion. In good accord with the drop weight impact sensitivity data, our calculations predict that the sensitivities of the molecules are very similar owing to the small variations of the energy output and rates of trigger linkage rupture. In addition, both the QMD and DFT calculations point to the nitropicramide N–NO2 bonds as the trigger linkages rather than the more typical O–NO2 bonds. We propose that the switch of the trigger linkage from the nitrate esters to the nitramine groups arises from the strongly electron withdrawing character of the adjacent trinitrobenzene groups.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


