一种选择性和口服生物可利用的RET降解剂在各种突变中有效的发现
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
转染期间重排(rearrange during transfection, RET)突变如G810C突变严重限制了选择性RET抑制剂在治疗RET驱动型癌症中的临床应用。本研究设计并评估了基于selpercatinib (LOXO-292)的RET蛋白水解靶向嵌合体(PROTACs),确定RD-23是一种有效的、选择性的RET PROTAC。RD-23能有效抑制多种RET突变的BaF3细胞的增殖,IC50值为2.4 ~ 6.5 nM。通过泛素-蛋白酶体系统选择性诱导RETG810C突变体降解,其DC50(引起50%蛋白质降解的浓度)值为11.7 nM。此外,与LOXO-292相比,RD-23在Ba/F3-KIF5B-RETG810C异种移植小鼠模型中表现出口服生物利用度和更好的抗肿瘤作用。这些结果表明,RD-23是治疗ret驱动型癌症的有希望的候选者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
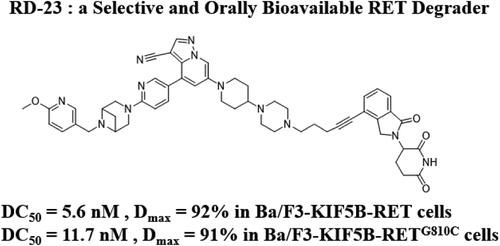
Discovery of a Selective and Orally Bioavailable RET Degrader with Effectiveness in Various Mutations
The rearranged during transfection (RET) mutation such as the G810C mutation has significantly restricted the clinical application of selective RET inhibitors in the treatment of RET-driven cancers. This study designed and evaluated RET proteolysis targeting chimeras (PROTACs) based on selpercatinib (LOXO-292), identifying RD-23 as a potent and selective RET PROTAC. RD-23 effectively inhibited the proliferation of BaF3 cells with various RET mutations, showing IC50 values of 2.4 to 6.5 nM. It selectively induced degradation of the RETG810C mutation via the ubiquitin–proteasome system, with a DC50 (concentration causing 50% of protein degradation) value of 11.7 nM. Additionally, RD-23 exhibited oral bioavailability and superior antitumor effects compared to LOXO-292 in a Ba/F3-KIF5B-RETG810C xenograft mouse model. These results suggested that RD-23 is a promising candidate for treating RET-driven cancers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


