Pt-Co单原子合金在水相中对糠醛加氢-重排串联反应的影响
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
水相串联反应作为绿色化学的一个基本方面,在当代精细化学品的合成中占有至关重要的地位,而高性能的非均相催化剂的发展仍然是一个艰巨的挑战。本文报道了一种Pt1Con单原子合金(SAA)催化剂,其中Pt单原子通过Pt - Co配位固定在Co纳米颗粒表面。Pt1Con SAA催化剂对糠醛(FAL)与环戊醇(CPL)的水相加氢重排反应具有较高的化学选择性(考虑碳损失,产率为93%),TOF值为2257 h-1(基于Pt)。基于反应动力学、同位素标记示踪实验、EPR和原位FT-IR的联合研究验证了cpl形成的五步连续串联反应途径。值得注意的是,在反应过程中,活性氢与水溶剂之间会发生氢原子的快速交换。此外,水分子不作为h给体,而是参与呋喃环侧链的重排反应。动力学研究结合DFT计算证实,Pt-Co界面位点通过促进羰基的活化吸附,有效降低了环戊酮(CPO)加氢步骤的能垒,从而大大增强了催化行为。本研究揭示了一种高效、稳定的异构催化剂在水相生物质升级反应中的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
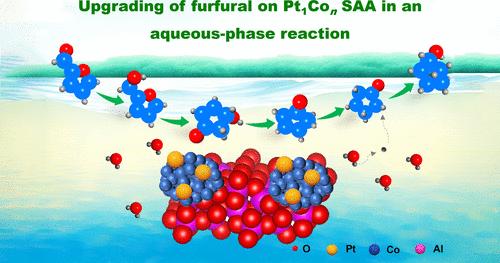
Pt–Co Single-Atom Alloy toward Furfural Hydrogenation–Rearrangement Tandem Reaction in the Aqueous Phase
Aqueous-phase tandem reactions, as a fundamental aspect of green chemistry, hold a crucial position in the contemporary synthesis of fine chemicals, wherein the advancement of high-performance heterogeneous catalysts remains a formidable challenge. Herein, we report a Pt1Con single-atom alloy (SAA) catalyst in which Pt single atoms are immobilized onto the surface of Co nanoparticles through Pt–Co coordination. The Pt1Con SAA catalyst exhibits a high chemoselectivity for the aqueous-phase hydrogenation–rearrangement reaction of furfural (FAL) to cyclopentanol (CPL) (yield: >93%, considering the carbon loss), with a TOF value of 2257 h–1 (based on Pt). A joint investigation based on reaction dynamics, isotope-label tracing experiments, EPR, and in situ FT-IR verifies a five-step consecutive tandem reaction pathway for the formation of CPL. Notably, during the reaction, the rapid exchange of hydrogen atoms would occur between activated hydrogen species and the water solvent. Furthermore, the water molecule does not serve as a H-donor but is involved in the rearrangement reaction in the side chain of the furan ring. Kinetic studies combined with DFT calculations substantiate that the Pt–Co interface sites effectively lower the energy barrier of the cyclopentanone (CPO) hydrogenation step via facilitating activation adsorption of the carbonyl group, accounting for the largely enhanced catalytic behavior. This study sheds light on the advancement of a highly efficient and stable heterogeneous catalyst for a biomass upgrading reaction in the aqueous phase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


