扩展晶格的亚稳立方间隙镍-碳体系的发现与表征
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
碳在铁中的间隙固溶体是亚稳的,即动力学上有利但热力学上不稳定。碳可以占据宿主金属的间隙原子,改变其性质。基体金属的合金化使FeCx相稳定,扩大了其应用范围。纯镍的应用主要集中在催化方面,而镍合金则广泛应用于燃气轮机、反应堆和海水管道。碳化镍(Ni3C)是众所周知的稳定的Ni-C体系,具有菱面体(R3′c)晶体结构。一些报道描述了一种难以捉摸的立方Ni-C体系,这种体系是在镍上发生的某些催化反应中观察到的,是由碳占据金属的间隙形成的。迄今为止,这种相的稳定和表征尚未完成。在此,我们报道了用甲烷化学气相沉积在负载镍纳米颗粒上合成立方亚稳NiCx相的方法。采用DFT/ReaxFF预测了该材料的结构,采用原位同步加速器XRD进行了合成和监测,并在环境条件下通过Rietveld细化和(S)TEM-EELS进行了实验验证。结果表明,在室温下,Fm3 - m相的晶格参数为a = 3.749±0.037 Å,碳原子占八面体间隙的原子百分比为23.1%,为NiC0.3相。碳占据间隙的程度可以被控制,从而可以调整宿主金属的d-间距和组成,突出了该合成路线在催化纳米颗粒制备中的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
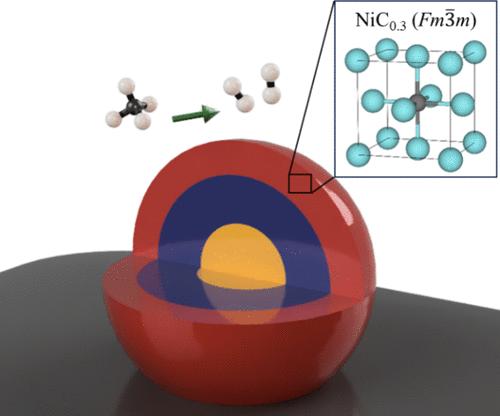
Discovery and Characterization of a Metastable Cubic Interstitial Nickel–Carbon System with an Expanded Lattice
Metastable, i.e., kinetically favored but thermodynamically not stable, interstitial solid solutions of carbon in iron are well-understood. Carbon can occupy the interstitial atoms of the host metal, altering its properties. Alloying of the host metal results in the stabilization of the FeCx phases, widening its application. Pure nickel finds niche applications, mainly focusing on catalysis, while nickel alloys are widely applied, e.g., in gas turbines, reactors, and seawater piping. Nickel carbide (Ni3C) is the well-known stable Ni–C system displaying a rhombohedral (R3̅c) crystal structure. Some reports describe an elusive cubic Ni–C system, observed during certain catalytic reactions occurring on nickel and formed by the occupation of the interstitials of the metal with carbon: to date, the stabilization and characterization of this phase have not been accomplished. Hereby, we report on the synthesis of a cubic metastable NiCx phase using chemical vapor deposition of methane on supported nickel nanoparticles. The structure was predicted by DFT/ReaxFF, synthesized and monitored with in situ time-resolved synchrotron XRD, and experimentally confirmed by Rietveld refinement and (S)TEM-EELS under ambient conditions. The results show an Fm3̅m phase with a lattice parameter of a = 3.749 ± 0.037 Å at room temperature, with the highest ever reported atomic percentage of carbon occupying the octahedral interstices of 23.1%, resulting in a NiC0.3 phase. The degree of occupation of the interstitial voids by carbon can be controlled, enabling the tuning of the host metal’s d-spacing and composition, highlighting the applicability of this synthesis route for catalytic nanoparticle preparation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


