Ag上单层MnI2岛的边缘能量驱动生长(111):高分辨率成像和理论分析
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
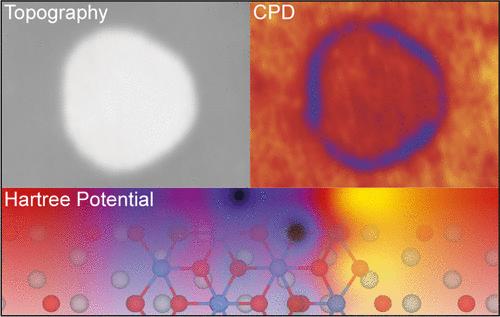
单晶表面上的过渡金属二卤化物薄膜的降维特性揭示了各种各样的磁性和电子特性。然而,实现化学计量单层岛需要精确控制生长条件。在这项研究中,我们使用扫描探针显微镜研究了MnI2在Ag(111)上通过单坩埚蒸发的生长。Ag(111)表面的催化性质促进了MnI2的脱卤,导致重建碘层的形成,作为截断六方MnI2岛生长的缓冲层。开尔文探针力显微镜显示,这些岛具有交替的边缘长度和不同的开尔文势。密度泛函理论(DFT)计算支持实验观察到的岛屿高度和晶格参数,并提供了对原始边缘和重建边缘形成能量的见解。边缘长度的不对称归因于边缘形成能量的差异,由边缘碘原子的位置(上或下)驱动,正如DFT所证实的那样。这种结构上的差异解释了在两种类型的岛边末端之间观察到的开尔文势的变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Edge-Energy-Driven Growth of Monolayer MnI2 Islands on Ag(111): High-Resolution Imaging and Theoretical Analysis
The reduced dimensionality of thin transition metal dihalide films on single-crystal surfaces unlocks a diverse range of magnetic and electronic properties. However, achieving stoichiometric monolayer islands requires precise control over the growth conditions. In this study, we employ scanning probe microscopy to investigate the growth of MnI2 on Ag(111) via single-crucible evaporation. The catalytic properties of the Ag(111) surface facilitate MnI2 dehalogenation, leading to the formation of a reconstructed iodine adlayer that acts as a buffer layer for the growth of truncated hexagonal MnI2 islands. These islands exhibit alternating edge lengths and distinct Kelvin potentials, as revealed by Kelvin probe force microscopy. Density functional theory (DFT) calculations support the experimentally observed island heights and lattice parameters and provide insights into the formation energies of both pristine and reconstructed edges. The asymmetry in edge lengths is attributed to differences in edge formation energies, driven by the position (up or down) of edge iodine atoms, as confirmed by DFT. This structural difference accounts for the observed variation in the Kelvin potential between the two types of island edge terminations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


