pd催化级联Aza-Claisen重排策略在室温下模块化合成亚砜酰脒
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了在室温下通过pd催化的级联aza-Claisen重排和亲核反应合成亚砜酰脒的简捷方法。以游离nh -亚砜亚胺和n -烯丙酰胺为模块,在高化学选择性模型和100%原子效率下生产预期的亚砜亚胺脒类衍生物。在这些温和的反应条件下,广泛的官能团可以很好地耐受,从而得到所需的产品,通常收率很高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
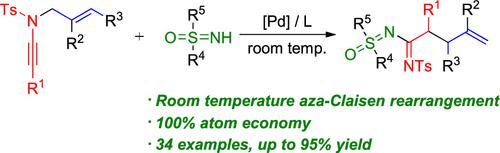
Modular Room Temperature Synthesis of Sulfoximidoyl Amidines Enabled by Pd-Catalyzed Cascade Aza-Claisen Rearrangement Strategy
Reported herein is a concise synthesis of sulfoximidoyl amidines enabled by a Pd-catalyzed cascade aza-Claisen rearrangement and nucleophilic reaction at room temperature. Free NH-sulfoximines and N-allylynamides were employed as the modular building blocks to produce the expected sulfoximine amidine derivatives in highly chemoselective models and in 100% atom efficiency. A broad range of functional groups were well tolerated under these gentle reaction conditions to give the desired products in generally good to excellent yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


