硅钨酸电子介质在v掺杂MoS2电催化剂上的析氢行为
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
金属掺杂硫化钼(MoS2)有望在电催化的析氢反应(HER)中实现类似铂的催化活性,尽管质子和电子转移动力学仍然知之甚少。在这项研究中,我们以硅钨酸为电子介质,研究了原始和钒(V)掺杂的MoS2催化剂的HER动力学和机理。v掺杂的MoS2显示出大约5倍于MoS2的HER率(696.4 vs 140.7 mmmolh2 g-1 min-1)。这种增强归因于质子吸附和转移动力学的加速,这是由Volmer步骤中活性位点数量的增加和有利的质子转移所驱动的。此外,硅钨酸通过球内机制介导电子转移,在原始和v掺杂的MoS2上表现出相同的反应顺序。电化学Tafel斜率表明,无论V掺杂与否,H2的演化都遵循Volmer-Heyrovsky机制。这项工作强调了质子吸附和转移动力学在提高HER速率方面的意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
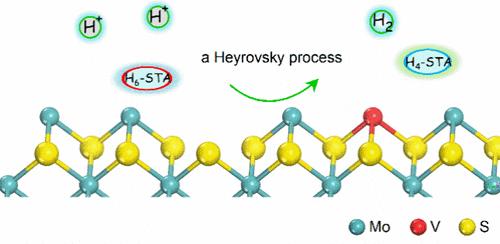
H2 Evolution with Silicotungstic Acid Electron Mediator over V-Doped MoS2 Electrocatalysts
Metal-doped molybdenum sulfide (MoS2) shows promise for achieving platinum-like catalytic activity in the hydrogen evolution reaction (HER) of electrocatalysis, though the proton and electron transfer kinetics remain poorly understood. In this study, we investigated the HER kinetics and mechanism on both pristine and vanadium(V)-doped MoS2 catalysts using silicotungstic acids as electron mediators. V-doped MoS2 exhibits an HER rate approximately 5 times that of MoS2 (696.4 vs 140.7 mmolH2 g–1 min–1). This enhancement is attributed to accelerated proton adsorption and transfer kinetics, driven by an increased number of active sites and favorable proton transfer in the Volmer step. Moreover, silicotungstic acids mediate electron transfer through an inner-sphere mechanism, showing identical reaction orders on both pristine and V-doped MoS2. Electrochemical Tafel slopes reveal that H2 evolution follows a Volmer–Heyrovsky mechanism, regardless of V doping. This work highlights the significance of proton adsorption and transfer kinetics in enhancing HER rates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


