探索生物质灰(BA)净化高富金属酸性矿井排水(AMDs):柱状和批状实验
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
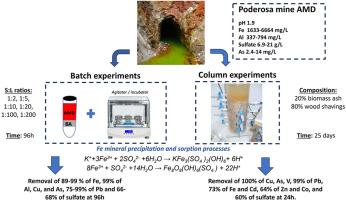
本研究探讨了生物质燃烧后产生的废弃物生物质灰(BA)作为碱性材料处理高酸性(pH 1.9-2.0)和富金属酸性矿山排水(AMD)的适用性。为了解决这个问题,分批处理(固液比1:2,1:5;1:10, 1:20, 1:100和1:200)和柱实验。在批量实验中,AMD与BA的接触引起了pH值的强烈增加,特别是在较高的S:L比(1:2和1:5)下,即分别从1.9到7.3和6.4,这是由于BA提供的碱度,这导致溶解的金属/固体(例如,89- 99%的Fe, 99%的Al, Cu和As, 75-99%的Pb)和硫酸盐(66-68%)在两种比例下都被强力去除。除As外,中低S:L比对大多数金属/固体的去除率显著较低,实验结束时所有S:L比的去除率均在95%以上。另一方面,最初包含在BA中的金属氧化物的溶解导致BA中常见的与这些氧化物相关的元素的释放(例如,Al, Ca, Mg, K, Na, Sr或P)。由于在实验的第一天碱度快速耗尽,在柱式实验中获得的去除率较低,这使得柱式反应器不适合AMD处理,而不是批式反应器。24h后Cu、As、V、Ga去除率为100%,Pb去除率为99%,Fe、Cd去除率为73%,Zn、Co去除率为64%,硫酸盐去除率为60%。然而,由于氧离子(H2AsO4-和HAsO42-)的优先吸附,到实验结束时,色谱柱的效率逐渐下降,除As(去除率约91%)外,达到与输入水中相似的值。在间歇式和柱式实验中,施魏锰矿和少量黄钾铁矾的沉淀和对这些矿物的吸附过程是控制金属保留的主要过程。尽管所使用的BA的碱度较低,但金属(类)s的去除率显著,因此,它构成了世界范围内产生这种废物的矿区处理AMD的有前途的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Exploration of biomass ashes (BA) to decontaminate highly metal-rich acid mine drainages (AMDs): Column and batch experiments
This work investigates the suitability of biomass ash (BA), a waste generated after biomass burning, as alkaline material to treat highly acidic (pH 1.9–2.0) and metal rich acid mine drainages (AMD). To address this issue, batch (at solid:liquid ratios of 1:2, 1:5; 1:10, 1:20, 1:100 and 1:200) and column experiments were performed. During batch experiments, the contact of AMD with BA provoked an intense increase of pH values, especially at higher S:L ratios (1:2 and 1:5), i.e., from 1.9 to 7.3 and 6.4, respectively, due to the alkalinity provided by BA, which led to strong removal of dissolved metal/loids (e.g., 89–99 % of Fe, 99% of Al, Cu and As, 75–99% of Pb) and sulfate (66–68%) for both ratios. The removal efficiency obtained using intermediate and low S:L ratios was remarkably lower for most metal/loids except for As, with values above 95% at the end of the experiment for all S:L ratios. On the other hand, the dissolution of metal oxides, initially contained in BA, led to the release of elements commonly found associated to these oxides in BA (e.g., Al, Ca, Mg, K, Na, Sr, or P). The removal rates obtained in column experiments were lower, due to the fast depletion of alkalinity during the first days of the experiment, which make columns less suitable for AMD treatment than batch reactors. A removal of 100% of Cu, As, V and Ga, 99% of Pb, 73% of Fe and Cd, 64% of Zn and Co, and 60% of sulfate was achieved after 24 h. However, the efficiency of the column decreased progressively to the end of the experiment, reaching similar values than in input waters, except in the case of As (around 91% of removal), due to the preferential sorption of oxyanions (H2AsO4− and HAsO42−). The precipitation of schwertmannite and to a lesser extent jarosite, and sorption processes on these minerals, are the main process controlling metal retention in both batch and column experiments. Despite the low alkalinity of the BA used, the removal rates of metal (loid)s were significant, and hence, it constitutes a promising option to treat AMD in mining areas worldwide where this waste is generated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


