起始枢纽在货物上的相分离是选择性自噬的触发开关
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
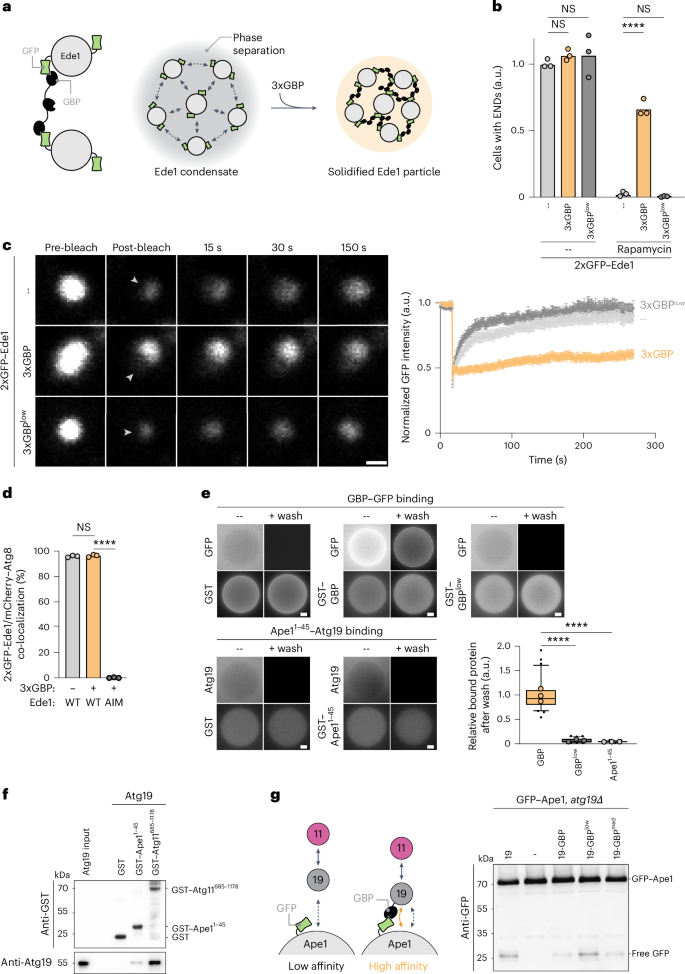
自噬是细胞质量控制的重要机制。营养应激触发大量自噬,自噬相关基因1 (Atg1)复合物的形成和液-液相分离使细胞质物质非选择性降解。相比之下,选择性自噬消除了蛋白质聚集体、受损的细胞器和其他被自噬受体靶向的物质。货物相分离已经被观察到,但其调控和对选择性自噬的影响尚不清楚。在这里,我们发现关键的自噬生物发生因子阶段在酵母的货物表面分离成起始中心,随后成熟为驱动吞噬细胞成核的位点。这种相分离依赖于自噬受体和货物之间的多价低亲和相互作用,从而产生动态货物表面。值得注意的是,自噬受体和货物复合物之间的高亲和力相互作用阻碍了起始中心的形成和自噬的进展。利用这些原理,我们将哺乳动物呼肠孤病毒非结构蛋白µNS转化为可通过选择性自噬降解的新货物,这种蛋白质在酵母细胞质中以颗粒形式积累,不被降解。我们发现起始中心也在人类细胞的不同载体表面形成,并且是建立与内质网连接的关键,内质网是形成吞噬体组装位点以启动吞噬体生物发生的地方。总的来说,我们的研究结果表明,在进化多样化的生物中,调节相分离强调了大量和选择性自噬的开始。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Phase separation of initiation hubs on cargo is a trigger switch for selective autophagy
Autophagy is a key cellular quality control mechanism. Nutrient stress triggers bulk autophagy, which nonselectively degrades cytoplasmic material upon formation and liquid–liquid phase separation of the autophagy-related gene 1 (Atg1) complex. In contrast, selective autophagy eliminates protein aggregates, damaged organelles and other cargoes that are targeted by an autophagy receptor. Phase separation of cargo has been observed, but its regulation and impact on selective autophagy are poorly understood. Here, we find that key autophagy biogenesis factors phase separate into initiation hubs at cargo surfaces in yeast, subsequently maturing into sites that drive phagophore nucleation. This phase separation is dependent on multivalent, low-affinity interactions between autophagy receptors and cargo, creating a dynamic cargo surface. Notably, high-affinity interactions between autophagy receptors and cargo complexes block initiation hub formation and autophagy progression. Using these principles, we converted the mammalian reovirus nonstructural protein µNS, which accumulates as particles in the yeast cytoplasm that are not degraded, into a neo-cargo that is degraded by selective autophagy. We show that initiation hubs also form on the surface of different cargoes in human cells and are key to establish the connection to the endoplasmic reticulum, where the phagophore assembly site is formed to initiate phagophore biogenesis. Overall, our findings suggest that regulated phase separation underscores the initiation of both bulk and selective autophagy in evolutionarily diverse organisms. Licheva, Pflaum, Babic, Mancilla et al. show that low-affinity cargo–receptor interactions promote autophagy receptor mobility and condensation of the scaffold protein Atg11 to trigger initiation hub and phagophore formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


