基于炎症生物标志物预测omalizumab在难治性慢性鼻窦炎伴鼻息肉合并哮喘患者中的有效性。
IF 4.3
2区 医学
Q2 ALLERGY
引用次数: 0
摘要
背景:基于临床评价,omalizumab治疗难治性慢性鼻窦炎伴鼻息肉(CRSwNP)已经得到了很好的研究。然而,迫切需要探索理想的定量或定性生物标志物来预测对生物制剂的不同反应。我们的目标是确定潜在的生物标志物,以预测难治性CRSwNP患者的良好或不良反应。方法:患者接受内窥镜和放射学评估,视觉模拟评分(VAS)评估和22项鼻窦结局测试(SNOT-22)。48种生物标志物,包括1型(T1)、2型(T2)和3型(T3)炎症因子、趋化因子和重塑因子,在基线和奥玛珠单抗治疗24周后在鼻分泌物和血清样本中检测到。结果:纳入18例CRSwNP患者和16例对照组。接受oamlizumab治疗的CRSwNP患者SNOT-22和VAS评分分别提高了8.9分和2分,分别为72.22%和50%。55.56%的患者鼻息肉评分(NPS)和Lund-Mackay评分明显改善。鼻分泌物和血清骨膜蛋白中T2炎症生物标志物、粒细胞-巨噬细胞集落刺激因子(GM-CSF)、T3炎症生物标志物、粒细胞集落刺激因子(G-CSF)、趋化因子(C-X-C基序)配体(CXCL)-1和趋化因子(C-C基序)配体-20、T1炎症生物标志物、IP-10 (CXCL-10)和颗粒酶B的浓度均显著降低。血清CCL-3 (AUC = 0.836)和CCL-4 (AUC = 0.909)水平分别预测SNOT-22评分的改善。血清IL-8 (AUC = 0.883)预测鼻塞评分改善不佳。鼻分泌物CXCL-1 (AUC = 0.812)、GM-CSF (AUC = 0.813)、IgE (AUC = 0.900)和IP-10 (AUC = 0.800)均能有效预测鼻息肉评分无或无改善。结论:Omalizumab显著影响不同途径的炎症介质。基于各种临床指标,血清中CCL-3和CCL-4以及IgE、CXCL-1、GM-CSF和鼻腔分泌物中的IP-10可能被认为是预测难治性CRSwNP合并哮喘患者对奥玛珠单抗治疗的有利或无效反应的较好生物标志物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Predicting the effectiveness of omalizumab in patients with refractory chronic rhinosinusitis with nasal polyps comorbid with asthma based on inflammatory biomarkers
Background
The treatment of refractory chronic rhinosinusitis with nasal polyps (CRSwNP) with omalizumab has been well studied based on clinical evaluation. Nevertheless, ideal quantitative or qualitative biomarkers for predicting a different response to biologics urgently need to be explored. We aim to identify potential biomarkers for predicting a good or poor response in patients with refractory CRSwNP.
Methodology
Patients received an endoscopic and radiological evaluation, a visual analogue scale (VAS) assessment, and a 22-item sinonasal outcome test (SNOT-22). Forty-eight biomarkers involving type 1 (T1), type 2 (T2), and type 3 (T3) inflammatory factors, chemokines, and remodeling factors were detected in nasal secretion and serum samples at baseline and after 24 weeks of omalizumab treatment.
Results
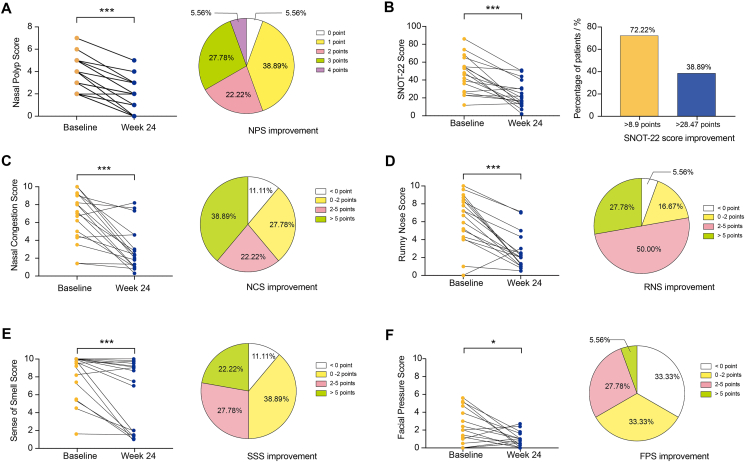
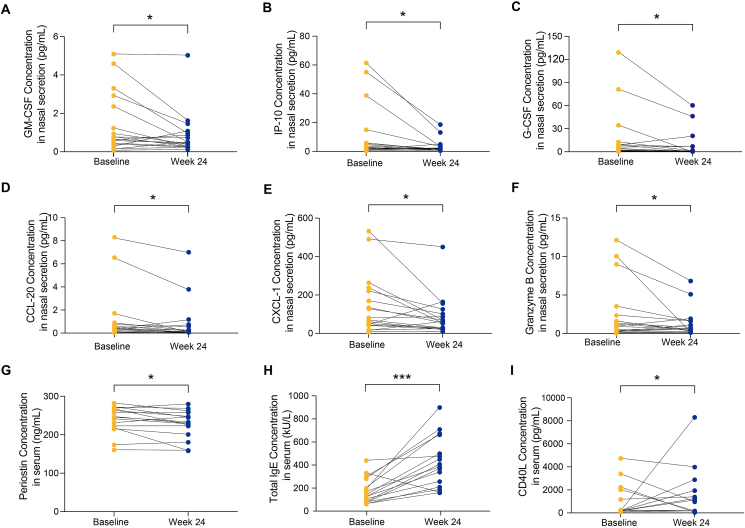
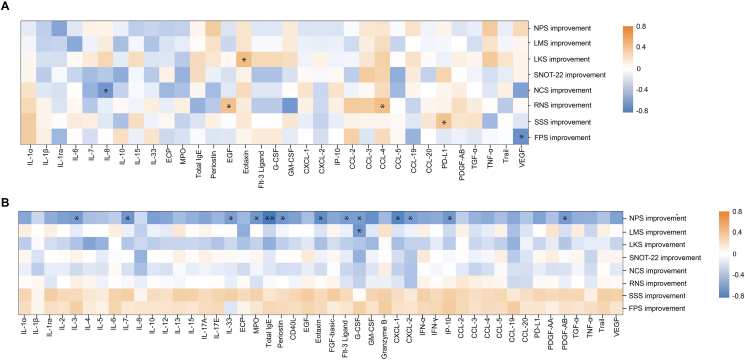
Eighteen patients with CRSwNP and 16 patients as control were enrolled. Patients with CRSwNP who received oamlizumab treatment with the SNOT-22 and VAS scores improved by 8.9 and 2 points in 72.22% and 50%, respectively. The nasal polyp score (NPS) and Lund-Mackay score were significantly improved in 55.56% of patients. The concentrations of T2 inflammatory biomarker, granulocyte-macrophage colony-stimulating factor (GM-CSF), T3 inflammatory biomarkers, granulocyte colony-stimulating factor (G-CSF), chemokine (C-X-C motif) ligand (CXCL)-1, and chemokine (C–C motif) ligand-20 (CCL-20), T1 inflammatory biomarker, IP-10 (CXCL-10), and granzyme B in nasal secretion and serum periostin were significantly decreased. Serum CCL-3 (AUC = 0.836) and CCL-4 (AUC = 0.909) levels predicted the improvement of SNOT-22 score, respectively. Serum IL-8 (AUC = 0.883) predicted poor improvement in nasal congestion score. Nasal secretion CXCL-1 (AUC = 0.812), GM-CSF (AUC = 0.813), IgE (AUC = 0.900) and IP-10 (AUC = 0.800) effectively predicted none or less improvement in nasal polyp score.
Conclusions
Omalizumab remarkably affects inflammatory mediators in different pathways. CCL-3 and CCL-4 in serum and IgE, CXCL-1, GM-CSF, and IP-10 in nasal secretion may be considered as preferable biomarkers for predicting favorable or ineffective response to omalizumab therapy in patients with refractory CRSwNP comorbid with asthma, based on various clinical indicators.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

World Allergy Organization Journal
Immunology and Microbiology-Immunology
CiteScore
9.10
自引率
5.90%
发文量
91
审稿时长
9 weeks
期刊介绍:
The official pubication of the World Allergy Organization, the World Allergy Organization Journal (WAOjournal) publishes original mechanistic, translational, and clinical research on the topics of allergy, asthma, anaphylaxis, and clincial immunology, as well as reviews, guidelines, and position papers that contribute to the improvement of patient care. WAOjournal publishes research on the growth of allergy prevalence within the scope of single countries, country comparisons, and practical global issues and regulations, or threats to the allergy specialty. The Journal invites the submissions of all authors interested in publishing on current global problems in allergy, asthma, anaphylaxis, and immunology. Of particular interest are the immunological consequences of climate change and the subsequent systematic transformations in food habits and their consequences for the allergy/immunology discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


